Leaves turning yellow, ice converting to water, milk turning to yogurt, candle melting, we observe a lot of changes in our surroundings on a daily basis. These changes are related to general Science and are mainly physical, or chemical in nature. However, it can get difficult to conclude whether the change is due to structural modification or due to chemical forces by just looking at the object. So, here we provide some key pointers which can help you in correctly distinguishing between physical and chemical changes!
This Blog Includes:
- What are the Physical Changes?
- What are Chemical Changes?
- Physical and Chemical Changes Examples
- Physical Changes Examples
- Chemical Changes Examples
- Difference between Physical and Chemical Changes
- Important Questions
- Is boiling water a chemical change?
- Which of the following is a physical change?
- Which of the following is not a physical change?
- Is dissolving a chemical change?
- Is evaporation a physical change?
- Is burning wood a chemical change?
- Is digesting food a chemical change?
- Is milk souring a chemical change?
- How is a chemical change different from a physical change?
- Is melting of wax a chemical change?
What are the Physical Changes?
Physical changes can be termed as those modifications which alter the way the molecules of a substance are arranged without affecting the molecular composition. In simple words, the object may appear or feel different, it does not lose its original chemical properties.
What are Chemical Changes?
Chemical changes, on the other hand, are those modifications that alter the molecular composition of a substance, thus creating an entirely new substance. The mediums undergo a chemical reaction which leads to a change in the atomic arrangement of its molecules.
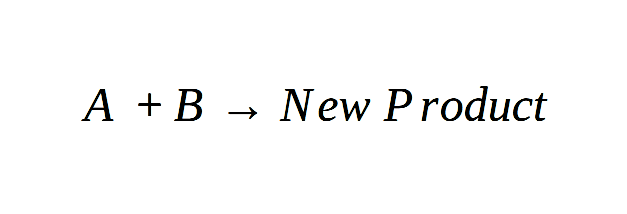
Physical and Chemical Changes Examples
To help you understand the concept of physical and chemical changes in a better way, we will first share some examples of physical alterations.
Physical Changes Examples
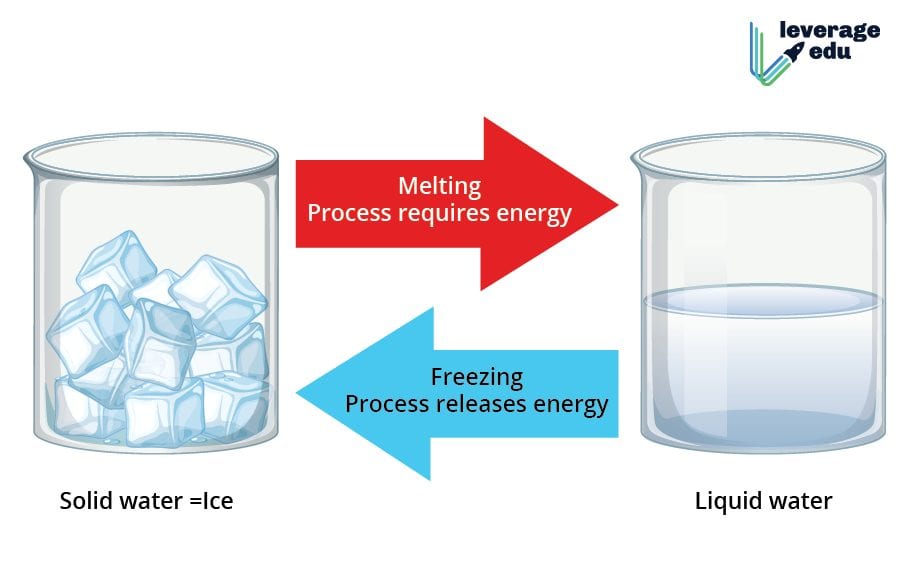
Melting of an Ice Cube
This is one of the most prominent examples of a physical change. If ice is exposed to high temperatures, it slowly melts down turning into water. What is interesting to note is that in ice, water molecules are packed closer together in solid form and upon melting, the molecules move freely. However, there are no changes in the molecular composition of water. The water can be frozen again to form ice, thus making the process reversible.
Chopping Wood
Chopping down wood essentially means cutting a piece of wood into smaller pieces. If we compare the physical and chemical changes for this example, then it is important to note that the change is entirely physical as the smaller pieces of wood still have the same molecular structure as before. Thus, the chemical structure remains intact.
Coke and Mentos
You must be familiar with the popular experiment of mixing coca-cola with mentos. When mentos is added to coca-cola, a carbonated drink, it results in a sudden surge of carbon dioxide trying to escape the bottle(brisk effervescence). Although this may look like a chemical change because of the release of gas, however, it is actually a physical modification. The escape of dissolved CO2 lead to no chemical change. Hence, this is an excellent example to elucidate the differences between physical and chemical changes!
Chemical Changes Examples
To make the concept of physical and chemical changes more clear, here are some important examples which show chemical modifications.
Electrolysis of Water
When an electric current is passed through water, a chemical change occurs thereby releasing Oxygen and Hydrogen gas. This is called electrolysis of water or famously known as, splitting of water. Here is a reaction that explains the process easily.
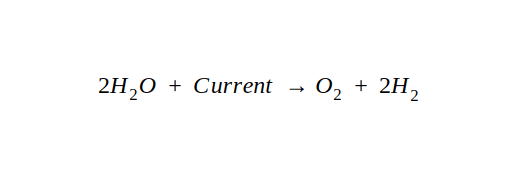
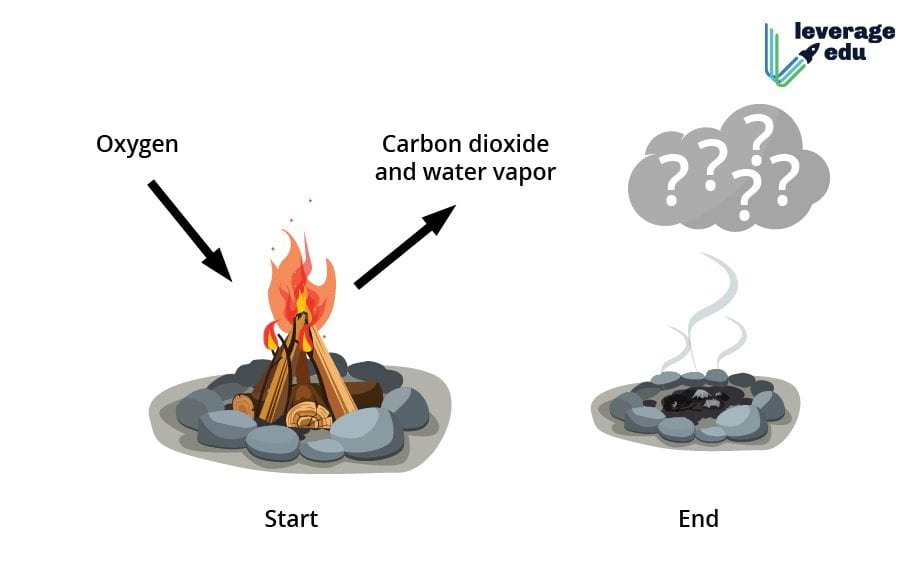
Burning of Wood
In contrast to the physical change of cropping wood, the burning of wood is a chemical change. Wood undergoes combustion in the presence of oxygen, emitting heat and turning into ash. Ash has an entirely different chemical composition and properties than wood. This is an irreversible chemical change as the ash produced by burning wood cannot be turned back into the wood.
Rusting of Iron
When citing examples of physical and chemical changes, this reaction has to be mentioned. Iron surfaces react with oxygen and water vapours present in the atmosphere to form rust, which are brittle, red-colored flakes. This weakens the substances and completely changes its chemical composition from a pure element to iron oxide. Rusting of a pipe is an ideal example.
Difference between Physical and Chemical Changes
Now that you have understood the basic concepts pertaining to both, let us summarise the differences between physical and chemical changes in a tabular form.
| Properties | Physical Changes | Chemical Changes |
| Reversibility | Physical changes are reversible | Chemical changes are generally irreversible but can be reversed in some cases using scientific processes |
| Formation of a new substance | No new substance is formed through physical changes | A chemical change always results in the formation of a new substance or change in the chemical composition of existing ones |
| Longevity | Generally temporary | Mostly permanent |
| Production of Energy | Physical changes do not produce energy | Chemical changes produces different types of energy |
| Modifications | Physical changes can only affect the shape and size of objects | Chemical changes can change both the physical and chemical properties of the substance |
Important Questions
Here are some of the important questions students ask or have about the physical and chemical changes:
Is boiling water a chemical change?
No, it is not a chemical change because the molecular structure of water vapor is the same as the liquid water i.e., H2O. Boiling water is an example of physical change.
Which of the following is a physical change?
- Electrolysis of Water
- Rusting of Iron
- Freezing of Water
Freezing of water is a physical change because it doesn’t change the molecular structure of the water and just changes the appearance.
Which of the following is not a physical change?
- Dissolving sugar and water
- Digestion of food
- Chopping wood
Digestion of food is a chemical change because for the body to absorb food macromolecules are converted to simpler molecules
Is dissolving a chemical change?
It depends on the substance you are dissolving. Dissolving a sugar does not form any chemical changes whereas dissolving a salt is a chemical change. Just like that dissolving an acid or any other ionic compounds are a chemical change.
Is evaporation a physical change?
Yes, this is a physical change because it doesn’t form any new substance through a chemical reaction.
Is burning wood a chemical change?
Yes because burning wood causes a chemical reaction and the formation of new substances such as coal, ashes, heat, or light.
Is digesting food a chemical change?
Yes, this is a classic example of a chemical change. For a human body to absorb food it is broken down into simpler substances from the macromolecules.
Is milk souring a chemical change?
Yes, souring changes the chemical properties of milk that result in acidification, and a new substance ferrous sulfide is formed.
How is a chemical change different from a physical change?
Physical changes appear different but don’t lose their chemical properties. On the other hand, chemical changes create a new substance. This just the basic difference between Physical and Chemical changes.
Is melting of wax a chemical change?
No, the melting of wax only changes the appearance of the wax once. When the wax starts cooling down it gets back to its original state.
These distinctions can help you in spotting the differences between physical and chemical changes easily! For reliable guidance in stream selection or taking the right step after class 12th, reach out to Leverage Edu experts. Not only will they help you in finding a suitable course but will also provide assistance in completing the admission procedure for universities abroad!
-
This article really helped me for my science test

 One app for all your study abroad needs
One app for all your study abroad needs





















 45,000+ students realised their study abroad dream with us. Take the first step today.
45,000+ students realised their study abroad dream with us. Take the first step today.


1 comment
This article really helped me for my science test