Class 11 chemistry syllabus includes many exciting and knowledgeable chapters. These chapters not only improve the general knowledge of the student but also lays the foundation for higher studies. Especially the chapter on Redox Reactions familiarize us with the intricate phenomenon of formation of a unique type of reaction. Being a chemistry student, you must go through the blog to explore some quirky details about class 11 redox reactions along with simple and effective notes for learning it.
This Blog Includes:
- What is Redox Reaction?
- Class 11 Chemistry Chapter 8 Redox Reactions Important Topics
- Redox Reactions in Reference With Electron Transfer Reactions
- Different Types of Redox Reactions
- What are Oxidation and Reduction?
- What is the Oxidation Number?
- How to Balance a Redox Reaction?
- Redox Reactions as the Basis for Titrations
- Application of Redox Reaction
- Redox Reaction Class 11 NCERT Solutions
- Practice Question on Class 11 Redox Reactions
What is Redox Reaction?
When the oxidation states of atoms are changed, and electrons get transferred between the two participating reactants, then such a type of chemical reaction is called a redox reaction. It is a combination of two reactions, i.e. oxidation and reduction reaction which involves electron transfer.
Under such a reaction, the oxidation state of the reacting chemical species gets changed, wherein one chemical species loses the electrons, and the other chemical species gains the electrons simultaneously.
For Example: A reaction between hydrogen and fluorine to form hydrogen fluoride. During the reaction, hydrogen oxidizes and loses two electrons, wherein fluorine gains two electrons.
Class 11 Chemistry Chapter 8 Redox Reactions Important Topics
Here are some of the important topics that you must study in this chapter:
- Redox Reactions in Reference With Electron Transfer Reactions
- Different Types of Redox Reactions
- Combination Reaction
- Decomposition Reaction
- Displacement Reaction
- Disproportionation Reaction(Dismutation)
- What are Oxidation and Reduction?
- What is the Oxidation Number?
- How to Balance a Redox Reaction?
- Redox Reactions as the Basis for Titrations
- Application of Redox Reaction
Redox Reactions in Reference With Electron Transfer Reactions
As per the topic class 11 redox reaction, a redox reaction is a combination of oxidation and reduction reaction that involves the movement of electrons. The reaction involves the loss of electrons and gain of electrons simultaneously which may happen in half proportion by establishing a correlation between the behaviour of electrons and its role in electron-transfer change.
Different Types of Redox Reactions
During a redox reaction, oxidation and reduction reactions take place simultaneously. Redox reactions further consist of four categories, i.e. Combination Reaction,
Decomposition Reaction, Displacement Reaction, and Disproportionation Reaction. Mentioned below is a detailed description of them-
Combination Reaction
A combination reaction is a type of reaction wherein two or more reactants, or compounds are combined to form a single compound or product. For Example, Calcium oxide is combined with water to form calcium hydroxide.
CaO + H2O → Ca(OH)2
Decomposition Reaction
A reaction wherein a single chemical compound is broken into two or more compounds is called a decomposition reaction. For Example, Hydrogen peroxide breaks into water and oxygen.
2 H2O2 → 2 H2O + O2
Displacement Reaction
A chemical reaction in which the more reactive element displaces the less reactive element from the compound. During this reaction, one or more than one atoms are replaced by other atoms. For Example, Iron displaces the copper metal when added to a copper sulphate solution.
Disproportionation Reaction(Dismutation)
As per class 11 redox reaction, dismutation is a type of redox reaction wherein one compound or reactant is oxidized and reduced. It converts the single compound into two compounds, in which one is of higher oxidation state, and the other one is of lower oxidation state. For Example, when heated, phosphorous acid disproportionates and gives phosphoric acid and phosphine.
4 H3PO3 → 3 H3PO4 + PH3
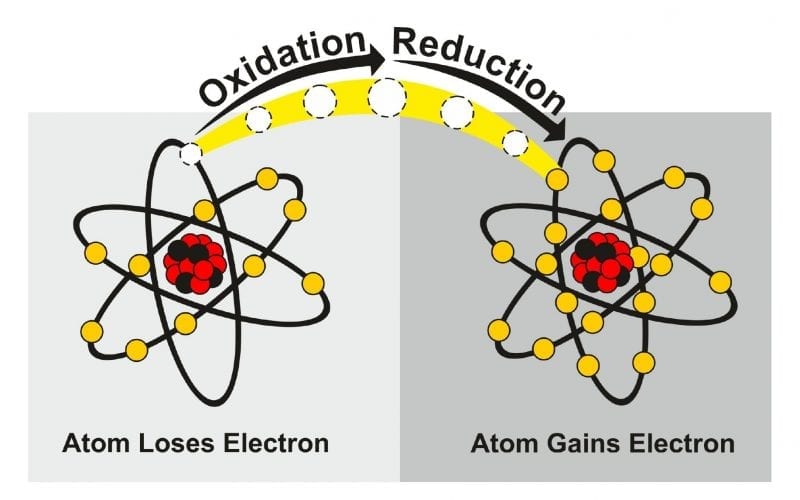
What are Oxidation and Reduction?
Oxidation is a type of reaction where an atom loses one or more electrons significantly. Under such a reaction, some elements may lose electrons easily and rapidly while other elements may require appropriate time and condition. Oxidation of elements occurs at this state. For Example, Iron metal oxidizes to form iron oxide (rust).
When a reactant gains one or more electrons during a chemical reaction then such reaction is called a reduction reaction. It always occurs in coexistence with the oxidation reaction only. Both the reactions are collectively called a redox reaction wherein one half is the reduction, and another half is the oxidation. For Example, Copper oxide and magnesium react to form copper and magnesium oxide.
CuO + Mg → Cu + MgO
What is the Oxidation Number?
The oxidation number is a method that allows keeping track of the shifts taking place in electrons due to the formation of covalent compounds during the chemical reaction. It formulates the rules based on electron pairs in a covalent bond and denotes the oxidation state of an element in a compound by referring it to electronegative elements. Oxidation number also states that in the free or uncombined state, the oxidation number of each atom will always be zero.
How to Balance a Redox Reaction?
As per the chapter on Redox Reactions for class 11, it is necessary to balance a redox equation. A redox reaction occurs with the help of an oxidizing agent and reducing agent. An oxidizing agent works to gain electrons while a reducing agent works to lose electrons. The oxidizing agent is often called an electron acceptor while the reducing agent is called an electron donor. Both the agents are required to balance a redox reaction.
A redox reaction can be balanced in two ways. The first method involves changing the oxidation number of oxidizing and reducing agents. In contrast, the other method involves dividing the redox reaction into two half-reactions, i.e. between reduction and oxidation. Here are some simple steps in which you can balance a redox reaction-
Step 1: Begin by writing the skeletal redox reaction carefully.
Step 2: indicate the exact number of oxidation of all the atoms given
Step 3: Find out the element(s) which have undergone oxidation reaction.
Step 4: For all the atoms, count the decrease or increase in the oxidation number.
Step 5: Equate the increase or decrease of the oxidation number on both sides of the reaction.
Step 6: Finally, balance the entire equation including the hydogen and oxidation atom.
Redox Reactions as the Basis for Titrations
Titration is a method that is used in the acid-base system to find the strength of one solution against another by using the pH sensitivity indicator. It can also be used in the redox reaction for finding out the strength of a reductant and oxidant using the redox sensitivity indicator. According to the topic class 11 redox reaction, it can also be used to restrict the reagents in the chemical reaction that are capable of oxidising the ions.
Application of Redox Reaction
There are numerous real-world applications of redox reactions that are useful in industrial processes and everyday life. For Example: Photosynthesis, Combustion, Electroplating, Electrolysis, etc.
- Chemicals like Caustic Soda and Chlorine are made using redox reactions
- Photosynthesis is a process that is used by green plants to convert water and carbon dioxide to carbohydrates. During photosynthesis, carbon dioxide gets reduced to form carbohydrates and water gets oxidized into oxygen with the help of a redox reaction
- Ammonia undergoes an oxidation reaction to form a nitric oxide which is an essential component of fertilizers
- Cutting an apple into half oxidizes it and changes the colour to brown
Redox Reaction Class 11 NCERT Solutions
Now that we are done with the theory part of the chapter, let us quickly go through some of the important practical questions to understand the chapter better.
Q. Assign oxidation number to the underline element of each of the following component-
- K2MnO4
- NaHSO4
Solution-
1. Let x be the oxidation number of Mn
Oxidation no. of K= +1
Oxidation no. of O= -2
Then,
2(+1) + x + 4(-2) = 0
2 + x – 8 = 0
x= +6
Therefore, the oxidation number of Mn is +6
2. Let x be the oxidation number of S
Oxidation no. of Na= +1
Oxidation no. of H= +1
Oxidation no. of O= -2
Then,
1(+1) + 1(+1) + 1 (x) + 4 (-2) = 0
1 + 1 + x – 8= 0
x= +6
Therefore, the oxidation number of S is +6
Q. Justify the following reactions-
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
Solution-
In the above reaction,
Oxidation no. of Fe and O in Fe and O in Fe2O3 is +3 and -2 respectively.
Oxidation no. of C and O in CO is +2 and -2 respectively.
Oxidation no. of Fe is 0.
Oxidation no. of C and O in CO2 is +4 and -2 respectively.
The oxidation no. of Fe decreased from +3 in Fe2O3 to 0 in Fe.
That is Fe2O3 is reduced to Fe.
The oxidation no. of C increased from 0 to +2 in CO to +4 in CO2
That is, CO is oxidized to CO2
Therefore, the reaction is a redox reaction.
4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (g)
In the above reaction,
Oxidation no. of N and H in NH3 is -3 and +1 respectively.
Oxidation no. of O2 is 0.
Oxidation no. of N and O in NO is +2 and -2 respectively.
Oxidation no. of H and O in H2O is +1 and -2 respectively.
The oxidation no. of N increased from -3 in NH3 to +2 in NO.
The oxidation no. of O2 decreased from 0 in O2 to -2 in NO and H2O. That is O2 is reduced.
Therefore, the reaction is a redox reaction.
Practice Question on Class 11 Redox Reactions
- Define oxidation reaction?
- In the reactions given below, identify the species undergoing oxidation and reduction:
H2S (g) + Cl2 (g) 2HCl (g) + S (S) - What is the most essential conditions that must be satisfied in a redox reaction?
- Define oxidation in terms of electron transfer.
- What happens to the oxidation number of an element in oxidation?
- The displacement reactions of Cl, Br, I using fluorine are not generally carried out in an aqueous solution. Give reason.
- Which gas is produced when less reactive metals like Mg and Fe react with steam?
- An electrochemical cell is constituted by combining Al electrode (E0 = – 1.66v) and Cu electrode (E0 = + 0.34v). Which of these electrodes will work as cathode and why?
- Balance the following equations by oxidation number method
CuO + NH3 Cu + N2 + H2O
K2 MnO4 + H2O MnO2 + KMnO4 + KOH
Thus, we hope that through this blog about class 11 redox reaction, you are through with an important topic of chemistry. Our career experts at Leverage Edu are here to help you formulate the best career-related decisions. Hurry up! Book an e-meeting!
-
It is good web site for notes
-
Hi Krish,
We are glad that our notes are helping you to study well. You can check out the important syllabus and notes for these subjects too for class 11:
https://leverageedu.com/blog/ncert-chemistry-class-11/
https://leverageedu.com/blog/physics-syllabus-for-class-11/
https://leverageedu.com/blog/ncert-class-11-maths-solutions/
-

 One app for all your study abroad needs
One app for all your study abroad needs




















 45,000+ students realised their study abroad dream with us. Take the first step today.
45,000+ students realised their study abroad dream with us. Take the first step today.


2 comments
It is good web site for notes
Hi Krish,
We are glad that our notes are helping you to study well. You can check out the important syllabus and notes for these subjects too for class 11:
https://leverageedu.com/blog/ncert-chemistry-class-11/
https://leverageedu.com/blog/physics-syllabus-for-class-11/
https://leverageedu.com/blog/ncert-class-11-maths-solutions/