The distinct forms in which matter can exist, are known as states of matter. You must be familiar with the basic properties of Solids, Liquids and Gases but with a slight change in the state of matter, the chemical properties and the corresponding reaction also gets affected. This is elucidated in detail in Chapter 5 of Class 11 chemistry syllabus. For those looking out for easy-to-understand study material for this chapter, we have compiled the complete summary and notes for class 11 States of Matter in this blog.
This Blog Includes:
Class 11 States of Matter NCERT PDF Download
States of Matter
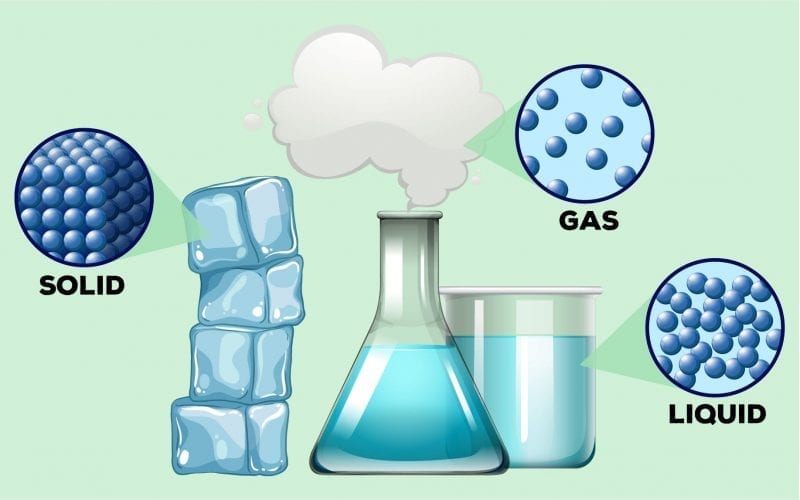
Mainly, the states in which a matter exists are Solid, Liquid, and Gas. Solids retain their fixed shape and volume, as the constituent particles are rigid and non-compressible. There are negligible interparticle spaces, and solids lack flowability. Gases and liquids are flowier in nature. They assume the size and shape of the container they are captured and the particles can freely move past each other because of the presence of a large number of interparticle spaces. Apart from this, there is another important state of matter known as Bose-Einstein Condensate where gases are cooled down to lower densities.
Intermolecular Forces
The forces of repulsion and attraction between the interacting particles are commonly referred to as Intermolecular Forces. These interacting particles have dipole moments that are permanent in nature. The attractive forces between molecules are known as the Van der Waal Forces, which varies with different types of intermolecular reactions. These varied forms of intermolecular forces, as discussed below:
Dispersion Forces or London Forces
The weakest intermolecular forces are known as Dispersion forces or London forces. It is a temporary attractive force that takes place when two adjacent electrons of bonded atoms form a temporary dipole by occupying positions. This is also referred to as induced dipole-dipole attraction as mentioned in the class 11 states of matter.
Dipole-Dipole Forces
This kind of attractive intermolecular force arises due to the positive end of one molecule reacting with the negative end of another reactive molecule. These attractive forces are only valid if the molecules are bonded very close together. They are weaker than covalent or ionic bonds. However, they are stronger than the London forces.
Induced-Dipole Forces
There are two different kinds of induced dipole forces such as ion-induced and dipole-induced forces. Ion-induced forces are weaker, and dipole-induced forces are stronger as in the states of matter. The resultant force from an ion-induced dipole with an atom or there is a disarrangement of protons in a nonpolar molecule. Similarly, dipole-induced forces result when a dipole is induced by a polar molecule in an atom or electron disarrangement in a nonpolar molecule.
Hydrogen Bond
The next topic in class 11 States of Matter is the hydrogen bond, the dipole-dipole interaction between a hydrogen atom with electronegative F, N, and O atoms in the form F-H, N-H or O-H bonds is known as hydrogen bonding. Chlorine (Cl) can also participate in the hydrogen bonding along with F, N, and O. Hydrogen bonding is vital when it comes to studying different characteristics of states of matter. There is a coulombic interaction between the hydrogen atom of one molecule with the lone-pair electrons of another electronegative atom of the reactive molecule.
States of Matter: Gaseous State and its Laws
The gaseous state is that state of matter in which there are large intermolecular spaces that exist between the particles. It is the simplest state of matter as stated by the class 11 chemistry chapter states of matter. Elements such as H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, and Rn exist as gases. It is important for human survival as it exists in the form of atmosphere. The laws governing gaseous states are as follows:
Boyle’s Law (Pressure-Volume ratio)
Stated by Robert Boyes, the law explains that the pressure of a fixed amount of gas is directly proportional to the volume of that gas, provided the temperature is constant.
Charles’s Law (Temperature-Volume ratio)
According to this law, the absolute temperature and volume of a gas are inversely proportional when the pressure of the gas is kept constant. Charles Law is necessary to study gaseous flow as per the states of matter chapter.
Gay-Lussac’s Law (Pressure-Temperature ratio)
The pressure and absolute temperature of a gas at constant volume and amount are inversely proportional to each other. The ratio of pressure and the temperature is constant.
Avogadro’s Law (Volume-Amount ratio)
Avogadro’s Law is one of the most important laws mentioned in class 11 states of matter. The number of atoms or molecules in a definite volume of a gas does not depend on gas’ molar mass or size.
Ideal Gas Equation
Coming to the next topic in our class 11 states of matter, the ideal equation for a gas explains that the equation for a gaseous state can be obtained by the collaboration of all these above-mentioned gaseous laws. An ideal gas equation explains the forces of gases and is known as the equation of states. This is not applicable for Bose-Einstein condensate.
According to Ideal gas Equation,
PV = nRT
where,
P = Pressure
V = Volume
n = Number of molecules
R = Gas constant
T = Temperature
States of Matter: Liquid State
The intermolecular forces are stronger in the liquid state than in the gaseous state. They are denser than gases and have less inter articular spaces in between them. The different properties of the liquid state of matter, as discussed in class 11 chemistry chapter states of matter are as follows:
Vapour Pressure
The vapour of a liquid exerts pressure known as vapour pressure. At a particular temperature, the dynamic equilibrium state is attained that determines the vapour pressure of the liquid at that particular temperature.
Surface Tension
Surface tension is the force that acts perpendicular per unit length at the surface of the liquid. Surface tension is inversely proportional to temperature as the forces per unit molecule usually decrease with the increase in the kinetic energy of the molecule.
Viscosity
[optin-monster-shortcode id=”xf2mlnjiouddzrshykdb”]The measurement of the resistance offered by the liquid while flowing is known as viscosity. Viscosity is directly proportional to the intermolecular forces of attraction, whereas it is inversely proportional to temperature. The liquids that flow at a slower rate have higher internal resistance, and on the other hand, the liquid that does not resist the flow has lower internal resistance.
Class 11 States of Matter NCERT Solutions
Source: Physics Wallah
Class 11 States of Matter PPT
Thus, we hope that through this insightful blog helped you understand the key pointers and summary of class 11 states of matter. Unsure about which career path to follow after 12th? Get in touch with our experts at Leverage Edu and we will help you sort out your choices and interests and find the right course and university to embark on this incremental step of your academic journey! Sign up for an e-meeting now!

 One app for all your study abroad needs
One app for all your study abroad needs




















 45,000+ students realised their study abroad dream with us. Take the first step today.
45,000+ students realised their study abroad dream with us. Take the first step today.

