Chemistry is often regarded as one of the favourite subjects of Class 12th students with science stream subjects. Bifurcated in two parts- Organic and Inorganic Chemistry, it encompasses a variety of essential topics like Chemical Kinetics, Electrochemical Series, Surface Chemistry, Solutions, p & f Block Elements etc. Thus, in order to nail the perfect score, you must strive to ace every topic of the subject. For all those who are gearing up for the class 12th chemistry, here is a blog on notes of the Electrochemical series.
This Blog Includes:
Electrochemical Series (NCERT) Definition
Activity series or electrochemical series is a list that comprises arrangements of elements in the order of increasing electrode potential values. By comparing and measuring the Standard Hydrogen Electrode (SHE) with respect to the potential electrodes, the series has been devised.
All the electrodes (metals and non-metals) as per their contact with their ions are arranged in the electrochemical series on the basis of their unique values of standard oxidation and reduction potential.
Now that you are familiar with the concept of the electrochemical series, let us understand the list in which the chemicals are arranged.
Electrochemical Series Chart
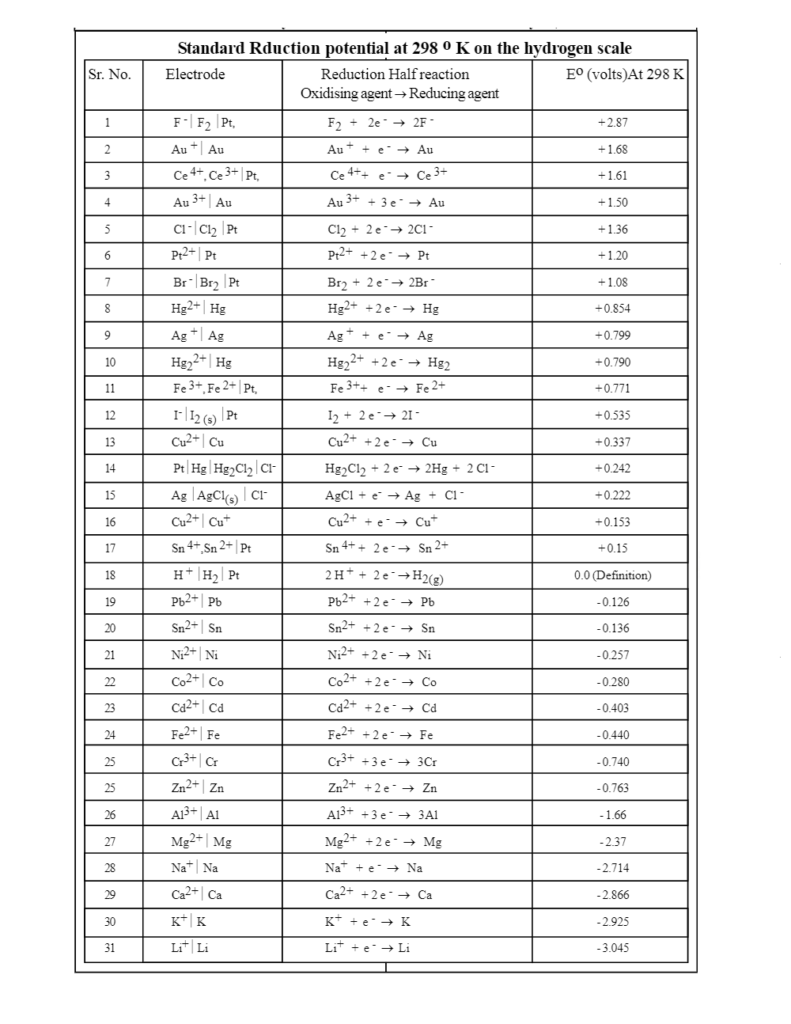
The image given below elucidates the activity series of the electrodes. The elements which are placed closer to each other, tend to display similar properties while those that far apart have various dissimilarities. In simpler words, in the electrochemical series, the metals which are distant from each other will have a higher propensity for corrosion as compared to ones that are near to each other. For a better understanding, let us have a look at this chart of activity series-

Electrochemical Series Table
Application of Electrochemical Series
The activity series may seem to be a simple arrangement of elements but it has various applications in the field of chemistry. Here are some applications of the same, exploring which you will have a better grip over the topic.
Have a look at Examples of Chemistry in Everyday Life
Oxidation and Reduction Strengths
By observing the electrochemical series, we can easily distinguish between the good oxidising agents and reducing agents. The oxidising agents possess the positive value of standard reduction potential and appear on the top of the series whereas the reducing agents are located at the bottom of the list and have a negative value of standard reduction potential.
For Instance: F2(+2.87) is a good oxidising agent and Li+(-3.05) is a strong reduction agent.
Now that you know so much about the Electrochemical Series, have a look at the list of Chemistry Project for Class 12 CBSE.
Standard emf (E0) of Electrochemical Cell
The standard emf of a cell is a combination of the standard reduction potential of the halves of the cell i.e., the oxidation half and reduction cell.
The standard oxidation potential is always determined in other terms of reduction potential
Hence,
standard oxidation potential ()= – standard reduction potential (
)
Also,
= [standard reduction potential of the reduction half of the cell ] – [standard reduction potential of the oxidation half of the cell]
Just like Electrochemical Series, there is so much to know about Basic Chemistry.
Determining the Feasibility of Redox Reaction
If the free energy change (G*) is negative, the redox reaction would occur spontaneously. The free energy of the cell emf is related to the following manner:
tG*= nFE*
n: Number of electrons evolved
F: Faraday constant
E*= Cell emf
Have a look at these pointers:
- If E* is positive then G* is negative
- The spontaneous reaction cannot take place if the E* turns out to be negative
- Only when the E* is positive, the reaction is negative and works as a source of electricity
- When a metal salt solution is stored in some other metal container, the value of E* for redox reaction is vital for determining its stability value
Must Read: How to Ace Chemistry Practical in Class 12th?
Finding the Product of Electrolysis
During the electrolysis, various metal ions are released at the electrodes when two or more negative and positive ions ate present in the solution. In general, the ion which is a good oxidising agent is released first at the cathode.
Must Read: Class 11 Chemistry Syllabus & Chapters
Electrochemical Series: Important Points To Remember
- The electrochemical series takes an element’s reduction potential in reference to the hydrogen scale, where Eo = zero. The definition of an element’s standard reduction potential is “the measure of an element’s likelihood to undergo reduction.”
- The larger an element’s reduction potential, the easier it is to reduce. Elements with a low reduction potential, on the other hand, will be oxidised considerably more quickly and easily.
- O n the other hand, elements with a negative or lower reduction potential, readily give up electrons. Elements with a positive or a greater reduction potential do not readily give away electrons but readily receive electrons.
- Stronger reducing agents with a negative standard reduction potential are typically found in the electrochemical series below hydrogen.
- In the series, however, weaker reducing agents with positive standard reduction potential are identified above the hydrogen.
- As we travel down the group, the strength of the reducing agent increases while the strength of the oxidising agent drops.
- Similarly, as we progress through the series, the electropositive and activity of metals increase or intensifies. It reduces in the case of nonmetals.
Electrochemical Series Trick
Hopefully, after reading this blog about the electrochemical series NCERT, you are all equipped with insights into this topic. Choosing the right career path after class 12th is the first step towards a successful career thus it is important to seek expert guidance for the same. Get in touch with Leverage Edu experts and they assist you towards a better future!

 One app for all your study abroad needs
One app for all your study abroad needs























 45,000+ students trusted us with their dreams. Take the first step today!
45,000+ students trusted us with their dreams. Take the first step today!


