Organic Chemistry is one of the branches of Chemistry that studies the structure, properties, composition, reactions and preparation of compounds containing carbon. These contain not just hydrocarbons but compounds with other elements like oxygen, nitrogen, phosphorus, halogens, sulfur and silicon. Initially, Organic Chemistry was limited to compounds produced by living organisms but it has now been stretched to human-made substances like plastics. Ranging from pharmaceuticals, food, explosives, petrochemicals to paints and cosmetics, application of such compounds is enormous. Class 12 Organic Chemistry includes 7 units contributing to nearly 28 marks in the CBSE class 12 Chemistry paper.
This Blog Includes:
NCERT Class 12 Organic Chemistry Chapters
Below is a list of units for Organic Chemistry as per the NCERT Chemistry Part II book:
- Unit VIII Haloalkanes and Haloarenes
- Unit IX Alcohols, Phenols and Ethers
- Unit X Aldehydes, Ketones and Carboxylic Acids
- Unit XI Organic Compounds containing Nitrogen (Amines)
- Unit XII Biomolecules
- Unit XIII Polymers
- Unit XIV Chemistry in Everyday Life
To know the latest CBSE structure and syllabus 2025, read our blog on Chemistry syllabus for class 12. You can see the mark distribution as per the important units and topics of the subject which has been asked in last year examination.
Also Read: Class 12 Physics Chapters
Important Concepts of Class 12 Organic Chemistry
Classification of Organic Compounds
Organic Compounds in class 12 Organic Chemistry have been broadly classified as follows:
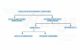
- Acyclic Compounds (Open-chain): Also known as aliphatic compounds, these consists of straight or branched chain compounds. E.g. Ethane, Isobutane, Acetic acid.
- Alicyclic Compounds (Closed-chain): These are aliphatic cyclic compounds consisting of carbon atoms attached in the form of a ring (homocyclic).
- Aromatic Compounds: E.g. Benzene, Aniline and Naphthalene, Furan, Thiophene, etc.
- Functional Groups: An atom or group of atoms combined in a specific manner that determines the chemical properties of an organic compound.
“Below, we have compiled some functional groups along with their examples, properties, and structures.
| Functional Group | Structure | Example | Properties |
| Alkyl | -C-H (e.g., -CH₃) | Methane (CH₄) | Non-polar, hydrophobic, often used in hydrocarbons. |
| Alcohol | -OH | Ethanol (C₂H₅OH) | Polar, can form hydrogen bonds, soluble in water. |
| Aldehyde | -CHO | Formaldehyde | Polar, reactive, often used in organic synthesis and as a preservative. |
| Ketone | -C=O (carbonyl group) | Acetone (C₃H₆O) | Polar, commonly used as a solvent in industrial processes. |
| Carboxylic Acid | -COOH | Acetic acid (CH₃COOH) | Polar, acidic, often found in organic acids. |
| Ester | -COO- | Ethyl acetate | Often fruity smells, used in perfumes and flavorings, derived from carboxylic acids and alcohols. |
| Amine | -NH₂ | Methylamine (CH₃NH₂) | Basic, nitrogen-containing, often used in pharmaceuticals and dyes. |
| Amide | -CONH₂ | Acetamide | Polar, often involved in protein structures, soluble in water. |
| Alkene | -C=C (double bond) | Ethene (C₂H₄) | Unsaturated, reactive, used in polymerization reactions. |
| Alkyne | -C≡C (triple bond) | Ethyne (C₂H₂) | Unsaturated, highly reactive, often used in welding. |
| Nitrile | -CN | Acetonitrile | Polar, commonly used as a solvent, and in synthetic processes. |
| Phenyl | -C₆H₅ | Benzene (C₆H₆) | Non-polar, aromatic, used in the production of plastics and chemicals. |
| Thiol | -SH | Methanethiol | Sulfur-containing, often have strong odors, can be toxic. |
Organic Chemistry: Haloalkanes and Haloarenes
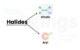
Classification of Haloalkanes and Haloarenes
- On the Basis of Number of Halogen Atoms: Compounds may be classified as mono, di, or polyhalogen compounds depending on whether they contain 1, 2 or more halogen atoms in their structures.

- Compounds Containing sp3 C—X Bond (X= F, Cl, Br, I): Alkyl halides or haloalkanes (R—X), Allylic halides and Benzylic halides.
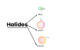
- Compounds Containing sp2 C—X Bond: Vinylic halides and Aryl halides.
Methods of Preparation
- From Alcohol: Alkyl chloride is prepared by either passing dry hydrogen chloride gas through a solution of alcohol or by simply heating a solution of alcohol in concentrated aqueous acid.
- From Hydrocarbons: By free radical halogenation (chlorination or bromination) of Alkanes, electrophilic substitution of arenes with chlorine and bromine and Sandmeyer’s reaction.
- From Alkenes: Addition of hydrogen halides and halogens
- Halogen Exchange: Finkelstein reaction (Reaction of alkyl chloride/bromide) with NaI in dry acetone to give out Alkyl iodides.
Chemical Reactions
- Reaction of Haloalkanes: Nucleophilic substitution, Elimination reactions and Reaction with metals.
- Reactions of Haloarenes: Nucleophilic substitution, Electrophilic substitution reactions and Reaction with metals (Wurtz-Fittig reaction and Fittig reaction)
In class 12 Organic Chemistry, you will study several polyhalogen compounds like chloroform, iodoform, dichloromethane, carbon tetrachloride, Freon and DDT (Dichlorodiphenyltrichloroethane) have many industrial applications. However, some of these cannot be decomposed easily resulting in depletion of the ozone layer and increasing environmental hazards.
Also Read: Biology Syllabus for Class 12
Organic Chemistry: Alcohols, Phenols and Ethers
Classification of Alcohols, Phenols and Ethers
- Mono, Di, Tri or Polyhydric Compounds
| Category | Type | Definition | Examples |
| Alcohols | Monohydric Alcohol | Alcohols containing one hydroxyl group (-OH) attached to a saturated carbon atom. | Methanol (CH₃OH), Ethanol (C₂H₅OH) |
| Dihydric Alcohol | Alcohols containing two hydroxyl groups (-OH) attached to different carbon atoms. | Ethylene glycol (C₂H₄(OH)₂) | |
| Trihydric Alcohol | Alcohols containing three hydroxyl groups (-OH) attached to three different carbon atoms. | Glycerol (C₃H₅(OH)₃) | |
| Phenols | Monohydric Phenol | Aromatic compounds with one hydroxyl group (-OH) attached directly to the aromatic ring. | Phenol (C₆H₅OH) |
| Dihydric Phenol | Phenols containing two hydroxyl groups (-OH) attached to the aromatic ring. | Catechol (1,2-dihydroxybenzene), Resorcinol (1,3-dihydroxybenzene) | |
| Trihydric Phenol | Phenols containing three hydroxyl groups (-OH) attached to the aromatic ring. | Pyrogallol (1,2,3-trihydroxybenzene) | |
| Ethers | Symmetrical Ether | Also known as simple ether, the alkyl or aryl groups attached to both sides of the oxygen atom are the same. | Dimethyl ether (CH₃-O-CH₃), Dipropyl ether (C₃H₇-O-C₃H₇) |
| Unsymmetrical Ether | Also known as mixed ether, the alkyl or aryl groups attached to both sides of the oxygen atom are different. | Methyl propyl ether (CH₃-O-C₃H₇), Propyl butyl ether (C₃H₇-O-C₄H₉) |
Monohydric alcohols in class 12 Organic Chemistry can be further classified into:
Compounds containing C sp3—OH Bond: Primary, secondary and tertiary alcohols, Allylic alcohols and Benzylic alcohols
| Type of Compound | Definition | Examples |
| Allylic Alcohols | Alcohols where the hydroxyl group (-OH) is attached to an sp³-hybridized carbon atom adjacent to a double bond (C=C). | Allyl alcohol (CH₂=CHCH₂OH), Crotyl alcohol (CH₃CH=CHCH₂OH) |
| Benzylic Alcohols | Alcohols where the hydroxyl group (-OH) is attached to an sp³-hybridized carbon atom adjacent to an aromatic ring. | Benzyl alcohol (C₆H₅CH₂OH), 2-Phenylethanol (C₆H₅CH₂CH₂OH) |
Compounds containing Csp2—OH Bond: Vinylic alcohol and Phenols

Ethers are classified as simple or symmetrical and mixed or unsymmetrical.
Also Read: Physics Syllabus for Class 12
Methods of Preparation
Preparation of Alcohols from
- Alkenes: By acid catalysed hydration and hydroboration–oxidation
- Carbonyl Compounds: By reduction of aldehydes and ketones and reduction of carboxylic acids and esters
- Grignard Reagents
Preparation of Phenols from
- Haloarenes
- Benzenesulphonic Acid
- Diazonium Salts
- Cumene (Isopropylbenzene)
Preparation of Ethers by
- Dehydration of Alcohols
- Williamson Synthesis
Chemical Reactions
- Reactions involving Cleavage of O—H Bond: Acidity of Alcohols and Phenols and Esterification
- Reactions involving Cleavage of C—O Bond in Alcohols: Reaction with hydrogen halides, Reaction with phosphorus trihalides, Dehydration Oxidation
- Reactions of Phenols: Electrophilic aromatic substitution, Kolbe’s reaction, Reimer-Tiemann reaction, Reaction of phenol with zinc dust, Oxidation
- Cleavage of C–O Bond in Ethers
- Electrophilic Substitution: Halogenation, Friedel-Crafts reaction and Nitration
Organic Chemistry: Aldehydes, Ketones and Carboxylic Acid

Methods of Preparation
Preparation of Aldehydes and Ketones by
- Oxidation of Alcohols
- Dehydrogenation of Alcohols
- Hydrocarbon: Ozonolysis of Alkenes and Hydration of Alkynes
Preparation of Aldehydes from
- Acyl Chloride (acid chloride)
- Nitriles and Esters: Stephen Reaction
- Hydrocarbons: Oxidation of Methylbenzene, Etard Reaction, Side-chain chlorination followed by hydrolysis and Gatterman – Koch reaction
Preparation of Ketones from
- Acyl Chlorides
- Nitriles
- Benzene or Substituted Benzenes: Friedel-Crafts acylation Reaction
Preparation of Carboxylic Acids from
- Primary Alcohols and Aldehydes
- Alkylbenzenes
- Nitriles and Amides
- Grignard Reagents
- Acyl halides and Anhydrides
- Esters
Chemical Reaction
- Nucleophilic addition reactions
- Reduction to alcohols and hydrocarbons (Clemmensen reduction and Wolff-Kishner reduction)
- Oxidation: Tollens’ test, Fehling’s test and Oxidation of methyl ketones by haloform reaction
- Reaction due to α-hydrogen: Acidity of α-hydrogen of aldehydes and ketones, Aldol condensation, Cross aldol condensation
- Other reactions: Cannizzaro reaction, Electrophilic substitution reaction
- Reactions Involving Cleavage of O–H Bond: Acidity
- Reactions Involving Cleavage of C–OH Bond: Formation of Anhydride, Esterification, Reactions with PCl5, PCl3 and SOCl2 and Reaction with Ammonia
- Reactions Involving –COOH Group: Reduction and Decarboxylation (Kolbe electrolysis)
- Substitution Reactions in the Hydrocarbon Part: Halogenation (Hell-Volhard-Zelinsky reaction), Ring substitution (Friedel-Crafts reaction)
Also Read: How to Ace Chemistry Practical in Class 12th?
Reference Books for Organic Chemistry
Many times students are unable to understand the bulky concepts of organic chemistry class 12trh from the formal language used in the NCERT books. In such a case, candidates need to refer to the right book to understand these concepts well. Here is a list of popular reference books for chemistry:
| Book | Buy Here |
| Modern ABC of Chemistry Class-12 Part I & Part II | Buy Here |
| All In One Physics CBSE Class 12 | Buy Here |
| Xam Idea Chemistry for CBSE Class 12 | Buy Here |
| 10 Years CBSE Champion Chapterwise-Topicwise – Chemistry-Class- 12 | Buy Here |
| Oswaal NCERT Exemplar (Problems – solutions) Class 12 Chemistry Book | Buy Here |
| thinkBoard: Class – 12 Board Companion Chemistry | Buy Here |
FAQs
Here are some tips to study 12th organic chemistry with ease:
– Be regular at studies
– Make sure you revise previous chapters on a daily basis
– Make a study plan
– Refer to the appropriate study guide
– Only cover topics mentioned in the NCERT books
Following are the chapters that are a part of class 12 organic chemistry:
– Aldehydes, Ketones and Carboxylic Acids
– Haloalkanes and Haloarenes
– Polymers
– Alcohols, Phenols and Ethers
– Amines
– Biomolecules
– Chemistry in everyday life
There are 28 basic reactions in organic chemistry class 12th. Students must learn the reactions along with the catalyst and end products.
If you practice chemistry every day and go through the reactions, formulas and concepts on a daily basis, then organic chemistry would be really easy and scoring for you!
With a detailed syllabus for class 12 Organic Chemistry, we hope you’ll prepare well for the upcoming CBSE Chemistry exam 2025. Organic Chemistry is, directly and indirectly, involved in various facets of everyday living. Whether it is developing new medicines, plastics, fuels or food, Organic Chemistry majors enjoy a diverse range of lucrative career opportunities. Is chemistry your subject of interest and you are planning to study abroad after 12th? Then, our experts at Leverage Edu can help you identify the right university that will provide you the foundation to fulfil your dream of making an amazing career out of the study of molecules, elements, compounds and chemical reactions.
-
Nice
-
Hi, Ram! Thank you for liking our blog. Keep yourself updated with our amazing content.
Here we are also providing you with some other blogs to read: Self and Personality Class 12 Notes
Attitude and Social Cognition Class 12 Notes
Accountancy Class 12 Notes
Balance of Payments Class 12 Notes
-
-
My thoughts is how to study in chemistry

 One app for all your study abroad needs
One app for all your study abroad needs



















 45,000+ students trusted us with their dreams. Take the first step today!
45,000+ students trusted us with their dreams. Take the first step today!



4 comments
Nice
Hi, Ram! Thank you for liking our blog. Keep yourself updated with our amazing content.
Here we are also providing you with some other blogs to read: Self and Personality Class 12 Notes
Attitude and Social Cognition Class 12 Notes
Accountancy Class 12 Notes
Balance of Payments Class 12 Notes
My thoughts is how to study in chemistry
very informative