Class 8 materials metals and non-metals, is chapter 4 of Science. It is a very interesting chapter that explains the different properties of metals and non-metals. To begin with, we learn about the physical properties of metals and non-metals such as malleability, ductility, conductivity, etc. Further, we explore their chemical properties. As we proceed, we will learn about the uses of metals and non-metals. You can use the notes to answer state board examinations, SSC, UPSC CSE prelims, and other government examinations with Science questions. Let us proceed!!
This Blog Includes:
Also Refer to CBSE Notes Class 6 Science
Revise CBSE NCERT Notes Class 7 Science
Physical Properties of Metals and Non-metals
Let us start the NCERT Class 8 Science chapter “Materials: Metals and Non-Metals” with the physical properties of different substances.
Malleability – The property by which they can be beaten into thin sheets is a characteristic property of metals and is called malleability.
Ductility – The property of metals by which they can be drawn into wires is called ductility.
Lustrous – The shine on the surface of metals is called lustre so we know that metals are lustrous.
Sonority – Metals produce ringing sounds and are said to be sonorous. Whereas, Non-metals are not sonorous.
Conductivity – Metal things like an iron rod, nail and copper wire are good conductors of heat and electricity while rolled sulphur piece and coal piece are poor conductors being non – metals.
Some exceptions – Metals like sodium and potassium are soft and can be cut with a knife. Mercury is the only metal that is found in a liquid state at room temperature.
Class 8 Cell Structure and Function Study Notes

Chemical Properties of Metals and Non-metals
Now, let us delve into the chemical properties of metals and non-metals as described in NCERT Class 8 Science Chapter on Materials.
Reaction with Oxygen – Iron rusts in the presence of oxygen or being exposed to moist air, magnesium similarly reacts to oxygen by burning. In both cases oxide formation takes place. Copper vessels when exposed to moist air for long acquire a dull green coating of copper hydroxide and copper carbonate represented by the formula 2Cu+H2O+CO2 +O2→Cu (OH)2 + CuCO3. So we find that burning metals react with oxygen to produce metal oxides which are basic in nature.
Sulphur’s reaction with oxygen makes sulphur dioxide gas. When sulphur dioxide is dissolved in water sulphurous acid is formed by the formula – Sulphur dioxide (SO2 ) + Water (H2O) → Sulphurous acid (H2SO3 ) Oxides of non-metals are acidic in nature generally.
Reaction with Water – Sodium metal is very reactive and when it reacts with oxygen and water a lot of heat is generated in the reaction. It is therefore generally stored in kerosene to prevent this mishap. Some other metals like iron react with water slowly and do not have such immediate reactions. Some metals react with water to produce metal hydroxides and hydrogen gas.
Non-metals usually do not react with water though they may be very reactive in the air such non-metals are stored in water. For example, phosphorus catches fire if exposed to air. To prevent the contact of phosphorus with atmospheric oxygen, it is stored in water since it is a very reactive metal.
Reactions with Acids – Non-metals generally do not react with acids but metals react with acids and produce hydrogen gas that burns with a pop sound. Copper does not react with dilute hydrochloric acid even on heating but it reacts with sulphuric acid easily. Metals react with acids to produce metal salts and hydrogen gas.
Reactions with Bases – As before the pop sound indicates the presence of hydrogen gas and metals react with sodium hydroxide to produce hydrogen gas. Reactions of non-metals with bases are way more complex. So we find that some metals react with bases to produce hydrogen gas.
Displacement Reactions – One metal displaces another metal from its compound in aqueous solutions. Zinc replaces copper with copper sulphate and that’s why the blue colour of copper sulphate disappears and a powdery red mass of copper is deposited at the bottom of the beaker.
It can be represented by this formula to make it easy for you to understand – Copper Sulphate (CuSO4) + Zinc (Zn) (Blue) → Zinc Sulphate (ZnSO4) + Copper (Cu) (Colourless) (Red). Zinc can replace copper but copper cannot replace zinc because a more reactive metal can replace a less reactive metal but a less reactive one cannot replace a more reactive metal. This is a rule that applies in these reactions since zinc is more reactive it can displace copper but vice versa cannot happen.
Class 8 Combustion of Flame Study Notes
Uses of Metals and Non-metals
Metals and non-metals are used in various things we use in our everyday lives and now you will be able to identify them after learning about them. Metals are generally used in making machinery, automobiles, aeroplanes, trains, satellites, industrial gadgets, cooking utensils, water boilers, etc because the properties they possess are suitable for being used in these things.
Non-metals like air are essential for our life which all living beings inhale during breathing, wood is used in furniture, paper is used in books and there are so many other non-metals we use in our daily routine which you can easily identify on your own.
Also Read: Coal and Petroleum Class 8 Notes
FAQs
Metals are materials that are hard, lustrous, malleable, ductile, sonorous and good conductors of heat and electricity. Examples of metals are iron, copper, aluminium, calcium, magnesium, etc.
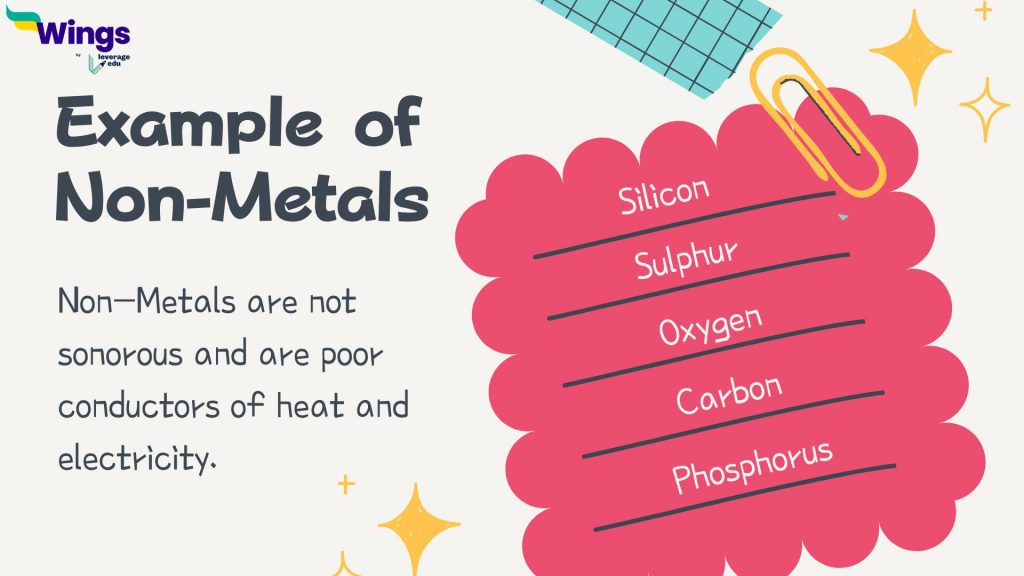
Non-metals are not sonorous and are poor conductors of heat and electricity. Examples of non-metals are sulphur, carbon, oxygen, phosphorus, etc.
The difference between metals and nonmetals is that metals are hard, lustrous, malleable, ductile, sonorous and good conductors of heat and electricity whereas non-metals are not. These are the basic differences between them.
Must Read: Science Projects for Class 8
Stay in tune with the school education page of Leverage Edu to access NCERT study material.

 One app for all your study abroad needs
One app for all your study abroad needs





















 45,000+ students realised their study abroad dream with us. Take the first step today.
45,000+ students realised their study abroad dream with us. Take the first step today.

