Whenever a physical body or a chemical molecule does some amount of work, it produces heat. The release of heat has several factors attached to it and the chapter 6 of class 11 chemistry syllabus elucidates the same. For the students preparing for their class 11 Chemistry examination or any other competitive exams like JEE mains or advanced, having a firm hold of chemistry concepts is crucial. So, let’s begin and get an insight into class 11 thermodynamics.
This Blog Includes:
- What is Thermodynamics?
- System and Surrounding
- Types of Systems
- Thermodynamic Properties
- State of System
- State Function
- Path Function
- Thermodynamic Process
- Laws Of Thermodynamics
- Internal Energy
- Mode of Transfer of Energy
- Heat Capacity
- Enthalpy
- Laws of Thermochemistry
- Entropy
- MCQs of Class 11 Thermodynamics
What is Thermodynamics?
Thermodynamics is the part of Chemistry that is concerned with the relations between work, heat, and different forms of energies.
System and Surrounding
System is the study of energy transfer and conversions into definitive space or area, whereas surrounding is defined as mass or region external to the system, or outside of it.
Types of Systems
There are basically 3 types of systems according to the class 11 thermodynamics chapter. Let us have a look at them-
Open System: It deals with the exchange of energy and matter between the system and region external to the system, or outside of it.
Closed System: In this type of system the exchange of matter cannot be made, but only energy can be switched between system and adjoining areas outside the system.
Isolated System: The type of system deals with no exchange of energies or matter between the system and surroundings
Thermodynamic Properties
There are two types of thermodynamic properties that are mentioned in the chapter. Before moving on with our notes, let us understand them
Extensive Properties: Defined as the properties whose values are affected by the mass of the total number of particles in the system and are categorized as extensive properties, e.g., Total energy, volume, etc
Intrinsic Properties: The type of properties that are affected by the concentrations and not on the mass of the total number of particles in the system. For example, Pressure, Density, Refractive Index, etc.
State of System
According to class 11 thermodynamics chapter, the condition of a system that is described in terms of certain observable or measurable properties such as Pressure, Volume, Temperature, etc is known as the state of system.
State Function
When variables of the function value depend only on the state of a system and are path independent by which the state has been attained, For Example: Potential energy Volume(V), Pressure(P), Temperature (T), Energy (U), Enthalpy (H), etc
Path Function
The value of the system which is dependent on the manner of transformations taken to establish property or value, e.g., heat, work, etc.
Thermodynamic Process
The transformation of a system from one equilibrium state to another is known as the thermodynamic process. According to class 11 thermodynamics chapter, it is of various types. Mentioned below are the few types-Isothermal Process: It is a process with no change in temperature
Isochoric Process
When the volume of the system doesn’t change, it will do no work on the surroundings.
Isobaric Process
It is a constant volume process. When the volume of the system doesn’t change, it will do no work on the surroundings.
Adiabatic Process
A thermodynamic process, where there is no transfer of heat between the system and region external to the system, or outside of it.
Cyclic Process
The thermodynamic Process, where the system comes back to its initial state after undergoing various transformations
Reversible Process
The reversible process takes place slowly and can be brought back to its original state without any trace on either system or surroundings.
Irreversible Process
The process that proceeds quickly in a single step and cannot be carried out in reversed order is called irreversible process.
Laws Of Thermodynamics
While exploring the thermodynamics chapter for class 11, you will come across a variety of laws. Here are the three laws of thermodynamics that you must learn along with their formulae to ace mathematical questions of the topic-
The First Law of Thermodynamics
The law states energy can neither be created nor be destroyed but can be converted to various forms.
Second Law Of Thermodynamics
The second law of thermodynamics states that entropy of the universe always expands in any spontaneous process. The spontaneous process cannot be brought back to its initial state.
Third Law of Thermodynamics
The third law of thermodynamics states an entropy of a perfectly crystalline compound is zero or constant when its temperature reaches equal or absolute zero:
T= 0 K is Zero
Internal Energy
As per class 11 thermodynamics chapter, internal energy can be defined as the sum of the total of all the energies possessed by the system. It is a state function. Internal Energy is denoted by U.
Here,
Q= Amount of heat given off to the system.
W= Work done
Mode of Transfer of Energy
As you know that neither energy can be created nor destroyed, it can only be transferred from one material to the other. There are basically two types to transfer the internal energy of a system.
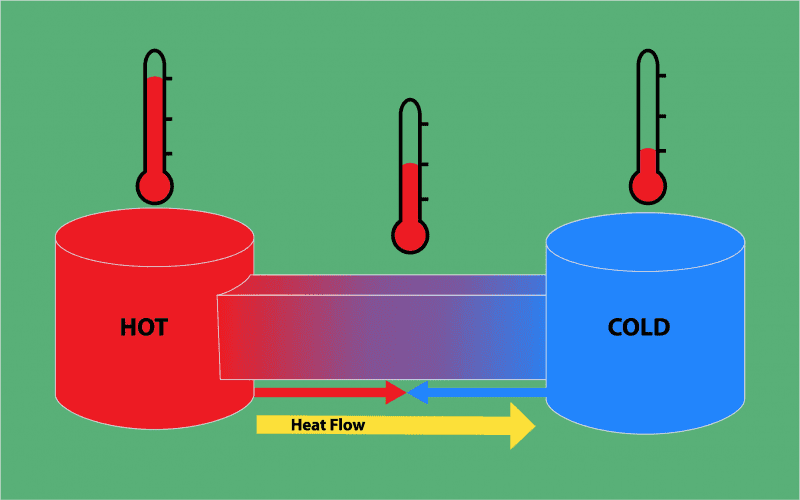
Heat: It is the energy transferred due to the temperature difference between the system and the surrounding, during heating kinetic energy of the molecule increases. Hence, internal energy increases.
Work(w): It is the energy spent to overcome an external force. When a system works against external pressure, then internal energy reduces and vice versa. Work is defined as pressure- work x volume (WpV).
Sign Conventions for Work and Heat
While studying the chapter, you will be required to use some sign conventions, such as-
- W is +ve when work is done on the system
- W is – ve when work is done by the system
- q is +ve when the heat is being absorbed by the system
- q is – ve when the heat is evolved by system
Work of Expansion or Compression
Work done in reversible isothermal Expansion of an ideal gas
Heat Capacity
It is defined as the quantity of heat that results in an increase of the system’s overall hotness or temperature by 1 degree Celsius. Here are some types of heat capacity-
- Molar Heat Capacity: The capacity of 1 mol of the substance in a system to take up the heat
- Specific Heat Capacity: Amount of energy needed to raise the temperature of 1g of the substance in a system
Q= mc Δ T
Enthalpy
It is defined as the amount of heat absorbed by the system to cause a change in the system. As per class 11 thermodynamics, it is an extensive property. Denoted by H i. e H = U + P. There are some forms of enthalpy reactions-
- Enthalpy of formation ΔHf: It is the change in enthalpy when 1 mole of a substance is formed under standard state from its pure elements
- Enthalpy of Combustion ΔHc: The enthalpy change that occurs when 1 mole of a substance is burnt completely due to the oxygen amount that is present
- Enthalpy of Solution: Enthalpy change that occurs when 1 mole of an ionic substance is diffused in water so that all ions get separated and do not interact with one another
- Enthalpy of Hydration: Enthalpy change that occurs when 1 mole of gaseous ions are dissolved into water to form 1mole of aqueous ions
Laws of Thermochemistry
The next chapter in our class 11 thermodynamics notes is the laws of thermochemistry. Let’s go through them-
Lavoisier Laplace Law
It states, the heat change in a forward reaction has the same magnitude of heat exchange in a backward reaction, but the sign it carries will be in the other direction.
Hess’s Law
Since Enthalpy is a state function, so the reaction taking place in steps can be calculated by adding the enthalpy change in each step. ΔH = ΔH1 + ΔH2 + ΔH3
[optin-monster-shortcode id=”xf2mlnjiouddzrshykdb”]Entropy
Entropy is defined as the computation of “degree of randomness” of energy in a system. It is a State function and an extensive Property. The SI unit of it is Jk – 1 mol-1. Entropy change in a reaction can be calculated by,
ΔrS° = Σ S° (products) – Σ S° (reactants) = qrev / T = ΔH / T
Where qrev = heat taken in by the system(reversible) and T= Temperature
MCQs of Class 11 Thermodynamics
a) The rate at which a reaction proceeds.
b) Energy changes involved in a chemical reaction.
c) The feasibility of a chemical reaction.
d) The extent to which a chemical reaction proceeds.
Ans: a
a) Temperature, amount, pressure
b) Pressure, volume, temperature
c) Pressure, volume, amount, temperature
d) Pressure, volume, temperature
Ans: c
a) Enthalpy of vaporization
b) Enthalpy of fusion + enthalpy of vaporization
c) Enthalpy of fusion
d) Twice the enthalpy of vaporization
Ans: b
C (g) + 4 H (g) —- CH4 (g): ΔR H = x kJ mol-1
C (graphite,s) + 2H2 (g) —- CH4 (g); ΔR H = y kJ mol-1
a) X = Y
b) X = 2Y
c) X < Y
d) X > Y
Ans: d
a) Gas expanding to fill the available volume
b) Gas in container contracting into one corner
c) Burning carbon in oxygen to give carbon dioxide
d) Flow of heat from colder to warmer body
Ans: a,c
Practice Worksheet of Class 11 Thermodynamics
Thus, we hope that through this blog about class 11 thermodynamics, you have understood the topic in detail. Are you exploring ways to get started on your dream career path? Reach out to our career experts at Leverage Edu and seek best guidance for future. Hurry up! Book an e-meeting.

 One app for all your study abroad needs
One app for all your study abroad needs




















 45,000+ students trusted us with their dreams. Take the first step today!
45,000+ students trusted us with their dreams. Take the first step today!


