Answer: Actinoid contraction is the gradual decrease in the atomic and ionic radii of actinoid elements. It occurs while moving from actinium (atomic number 89) to lawrencium (atomic number 103) in the periodic table. This contraction happens due to the poor shielding effect of 5f electrons. The nucleus attracts the outer electrons more strongly, pulling them inward and reducing the atomic size.
Complete Answer:
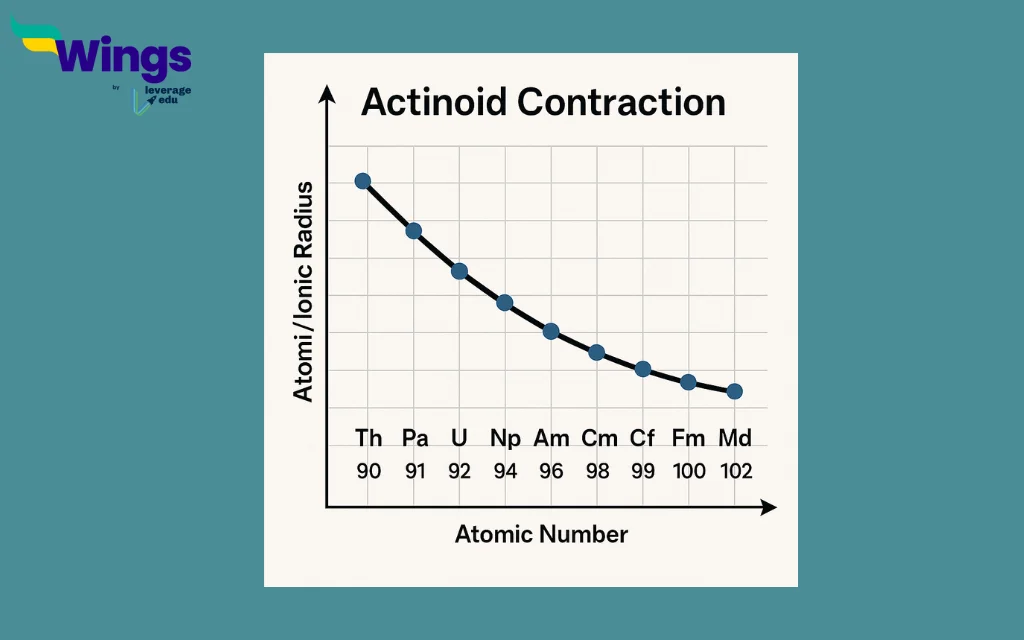
Actinoid contraction is the steady and consistent decrease in the atomic and ionic sizes of actinoid elements from actinium (89) to lawrencium (103) in the periodic table. Although the atomic number increases across the series, the atomic size does not increase; instead, it decreases due to the weak shielding effect of the 5f electrons.
The 5f electrons are progressively added as we move across the actinoid series. These electrons are more diffused and less effective at shielding the increasing positive nuclear charge. Consequently, the outer electrons experience a stronger pull from the nucleus, leading to a contraction in size. This phenomenon is called actinoid contraction.
Reasons for the Contraction of Actinoids
The main factors responsible for the gradual decrease in atomic and ionic sizes across the actinoid series, known as actinoid contraction, are as follows:
a. Poor Shielding by 5f Electrons
The 5f electrons are diffused and do not shield the nuclear charge effectively. Unlike s, p, or even d orbitals, the 5f orbitals are more spread out, allowing the increasing nuclear charge to pull the electrons inward. This results in a steady decrease in the size of atoms and ions across the actinoid series.
b. Increasing Nuclear Charge
As the atomic number increases across the actinoid series (from actinium to lawrencium), the number of protons in the nucleus also increases. This stronger positive charge pulls electrons closer, especially because the 5f electrons cannot efficiently block this attraction.
c. Greater Penetration of 5f Orbitals
The 5f orbitals penetrate closer to the nucleus compared to 4f orbitals. This leads to a stronger interaction with the nucleus and contributes to the overall reduction in size.
Consequences of Actinoid Contraction
Important effects of actinoid contraction on the chemical behaviour, properties, and periodic trends of the actinoid elements and the elements that follow them are:
a. Similarities in Chemical Properties
Due to the gradual size reduction, many actinoid elements have similar ionic sizes, especially in the +3 oxidation state. This leads to very similar chemical properties and often makes their separation difficult.
b. Complex Compound Formation
The smaller, highly charged ions resulting from actinoid contraction possess high polarising power. This enables actinoid elements to form stable and diverse coordination complexes with various ligands.
c. Variable Oxidation States
Unlike the lanthanoids, actinoids often exhibit multiple oxidation states. The contraction increases the effective nuclear charge, which helps stabilise different oxidation states across the series.
d. Impact on Later Elements in the Periodic Table
Similar to lanthanoid contraction, actinoid contraction affects the properties of elements that follow the actinoids in the periodic table. It influences trends in electronegativity, ionisation energy, and chemical reactivity.
Fun Fact
Despite the gradual decrease in size due to actinoid contraction. Actinoid elements show a wide variety of chemical behaviours and oxidation states. This makes them some of the most chemically versatile elements in the periodic table.
Check out other Chemistry questions from here:


 One app for all your study abroad needs
One app for all your study abroad needs











 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!