Learn how mind maps can help you remember key concepts and make connections between them and other relevant topics in JEE 2024. Discover how to create mind maps and their practical applications for the JEE exam. Using mind maps for the JEE exam is a type of visual learning aid that helps students organise and integrate difficult concepts and ideas clearly and methodically. They are particularly useful for JEE physics, chemistry and maths, which calls for a thorough comprehension of complex ideas and their relationships.
Contents
- 1 What is a Mind Map?
- 2 Why Subject-wise Mind Maps are Useful for JEE?
- 3 How to Create a Mind Map for JEE?
- 4 Tips for Optimizing Mind Map for JEE
- 5 Examples of JEE Mind Maps
- 6 Mind maps for JEE 2024 Maths Preparation
- 7 Mind maps for JEE 2024 Physics Preparation
- 8 Mind Maps for JEE 2024 Chemistry Preparation
- 9 FAQs
What is a Mind Map?
By controlling how your brain absorbs information, mind mapping is a technique that helps you organise the knowledge you remember so you can learn and know where to get it. Every mind map has a similar structure, with a central idea or concept from which several branches, each with its own branches, grow. There are many ways to make mind maps; you can use forms, colours, symbols, and lines to build “maps” of your ideas. A few coloured pens, your imagination, and a piece of blank paper large enough to accommodate your map will work just well. You can remember a map more easily if it is visually appealing, but this doesn’t mean you have to take your time making it seem good.
Why Subject-wise Mind Maps are Useful for JEE?
Subject-wise mind maps for JEE are a powerful tool for students preparing for the exam. Here’s why:
- Visual Representation: Mind maps present information visually, making it easier to see connections between concepts, which is especially helpful for Maths, Chemistry, and Physics.
- Simplified Learning: They break down complex information into manageable chunks, making learning more efficient.
- Comprehensive Coverage: Mind maps cover the entire JEE syllabus, ensuring students have all the necessary information in one place.
- Revision and Retention: Mind maps act as excellent revision tools, aiding memory retention through visual cues.
- Time-Saving: They condense complex topics concisely, saving students time for practice and focusing on weak areas.
How to Create a Mind Map for JEE?
Creating a mind map involves starting with a central idea and branching out with related concepts, using keywords, colours, and images to organize information visually. Here we have explained each of the steps to create last-minute revision mind maps for JEE in detail:
- Central Idea: Start with a central idea, keyword, or topic at the centre of your mind map.
- Main Branches: Brainstorm main branches radiating outward, representing primary categories. Label them clearly.
- Sub-Branches: Extend sub-branches from each main branch for more specific details and ideas. Label them concisely.
- Keywords & Visuals: Use keywords, short phrases, or clipart to represent ideas within each branch. Color-code for better organization.
- Cross-Linking: Find connections between sub-branches and use lines (different colors) to show how ideas interconnect.
Tips for Optimizing Mind Map for JEE
Optimize your mind map by keeping it focused, using concise keywords, incorporating colour coding, and using images for better understanding. Follow these steps for optimizing your last-minute revision mind map for JEE:
- Keep It Simple: Focus on the central idea and immediate branches. Avoid overcrowding with too many details. A clean mind map is best for brainstorming.
- Use Color Codes: Assign different colours to branches and categories to enhance visual organization and help your brain identify relationships.
- Prioritize Hierarchy: Arrange the main branches hierarchically, with the most important ideas closest to the Main Topic. This ensures critical information stands out.
Examples of JEE Mind Maps
JEE Mind Maps can act as your ultimate study companion, designed to help you conquer the JEE exam with confidence. Through the innovative approach of mindmaps for JEE, students can remember chunks of information for a longer time. Below, we have given a sample of how one can create a mind map from the basic information in the text. Suppose we read Rutherford’s atomic model from our NCERT books. After reading the original text 2-3 times, we must note the information on a rough sheet. These steps will help with better retention of the information. Once we are clear about the features of the Rutherford atomic model, we must then try to create a mind map based on the following information that we can recall:
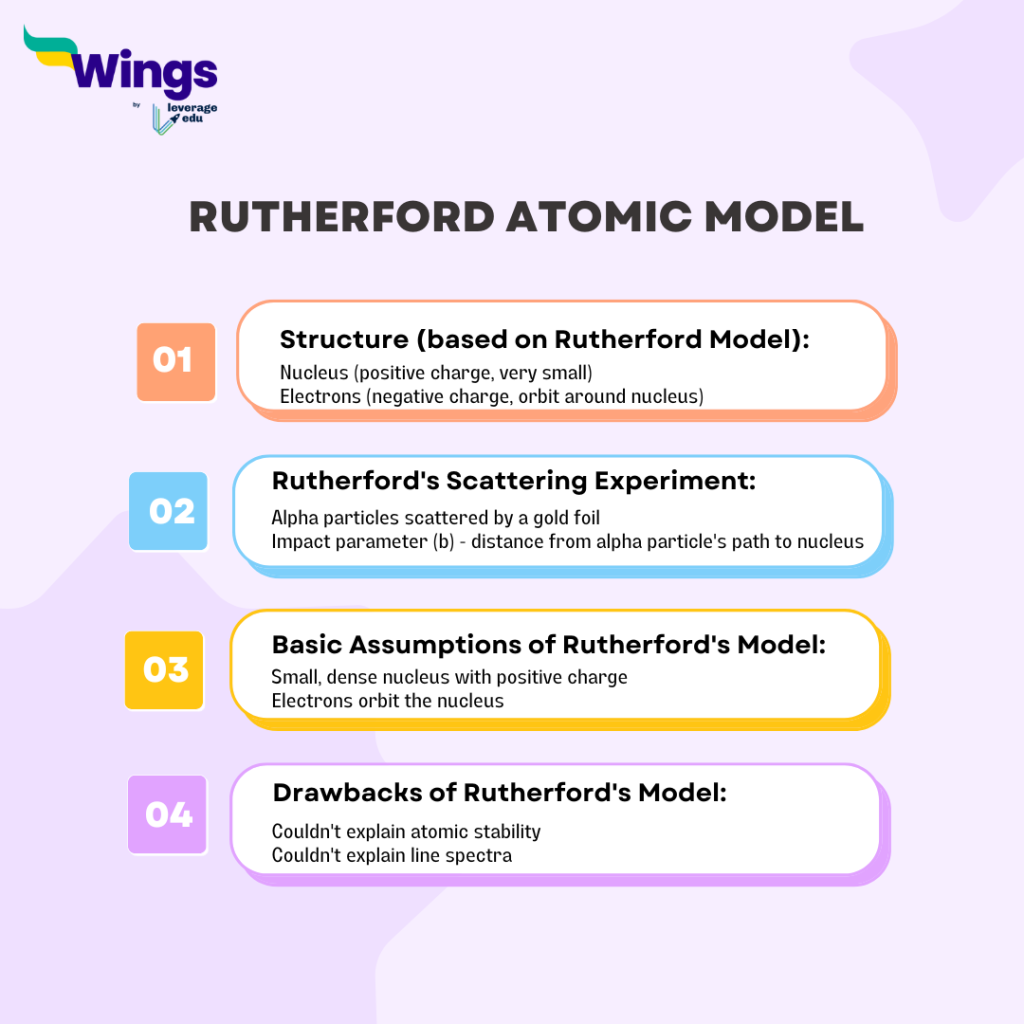
Mind maps for JEE 2024 Maths Preparation
Maths JEE Mind Maps streamline very important topics, organising formulas, concepts, and problem-solving methods into visual form, increasing comprehension, and ensuring exam readiness for improved performance.
Probability
- Definition
- The measure of the likelihood of an event to occur (0 to 1)
- Used to predict how likely events are to happen
- Formula
- P(E) = Favourable Outcomes / Total Outcomes
- Examples
- Coin toss (Heads/Tails)
- Coloured bottles experiment
- Tree Diagram
- Visualizes possible outcomes and their probabilities
- Used to determine addition and multiplication rules
- Types of Probability
- Theoretical Probability
- Based on reasoning and logic (e.g., coin toss = 1/2)
- Experimental Probability
- Based on observations from experiments (e.g., coin toss 6 heads in 10 tries = 3/5)
- Axiomatic Probability
- Set of rules (Kolmogorov’s axioms) to quantify chance of events
- Theoretical Probability
- Events
- Probability of an Event (P(E))
- Chance of event happening (r out of n possible outcomes)
- P(E) = r/n
- Probability of Event Not Happening (P(E’))
- Chance of event not happening
- P(E’) = 1 – P(E) = (n-r)/n
- Equally Likely Events
- All outcomes have the same probability (e.g., rolling a die)
- Complementary Events
- Two outcomes where only one can happen (e.g., rain or no rain)
- Probability of an Event (P(E))
- Probability Theory
- Branch of mathematics dealing with event possibilities
- Uses axioms to define probability within a sample space (0 to 1)
- Probability Density Function (PDF)
- Probability function for continuous random variables
- Used to understand normal distribution (mean and standard deviation)
Mind maps for JEE 2024 Physics Preparation
JEE Mind Maps Physics condenses complex concepts into visual diagrams for efficient revision, helping in understanding and enhancing exam performance. For example, we tried to condense the information related to electricity in mind maps format for your quick revision:
Electricity
- What is Electricity?
- Movement of charged particles (electrons)
- Static vs. Dynamic Electricity
- Static: Build up of electrical charge on object’s surface (no current flow)
- Caused by rubbing non-conductors (e.g., plastic, glass)
- Imbalance of charges creates attraction or repulsion
- Dynamic: Electrons in motion (current flow)
- AC (Alternating Current)
- DC (Direct Current)
- Static: Build up of electrical charge on object’s surface (no current flow)
- Electron Movement
- Valence electrons determine electrical properties
- Conductors, Semiconductors, Insulators
- Conductors
- Many free electrons (loose outer orbit)
- Examples: Gold, Copper, Silver
- Insulators
- Few or no free electrons
- Examples: Glass, Ceramic, Plastic
- Semiconductors
- In between conductors and insulators
- Examples: Silicon, Germanium
Mind Maps for JEE 2024 Chemistry Preparation
JEE Mind maps for Chemistry simplify lengthy topics into visual forms, helping in comprehension and increasing exam readiness for improved performance. Below we have created the sample mind map for ammonium chloride topic
Ammonium Chloride
- Structure
- Chemical Formula: NH4Cl
- Properties
- Molecular Weight/Molar Mass: 53.491 g/mol
- Density: 1.53 g/cm³
- Boiling Point: 520 °C
- Melting Point: 338 °C
- Appearance: Crystalline white solid
- Solubility: Highly soluble in water
- Acidity: Mildly acidic (pH 4.6 – 6.0 in 5% solution)
- Preparation
- Reaction of ammonia (NH3) with hydrochloric acid (HCl)
- NH3 + HCl → NH4Cl
- By-product of the Solvay Process
- CO2 + 2 NH3 + 2 NaCl + H2O → 2 NH4Cl + Na2CO3
- Reaction of ammonia (NH3) with hydrochloric acid (HCl)
- Chemical Reactions
- Decomposes to ammonia gas and hydrogen chloride
- NH4Cl → NH3 + HCl
- Reacts with sodium hydroxide (NaOH) to produce ammonia gas (NH3)
- NH4Cl + NaOH → NH3 + NaCl + H2O
- Reacts with sodium carbonate (Na2CO3) to produce sodium chloride (NaCl) and ammonia gas (NH3)
- 2 NH4Cl + Na2CO3 → 2 NaCl + CO2 + H2O + 2 NH3
- Decomposes to ammonia gas and hydrogen chloride
- Uses
- Fertilizer (nitrogen source)
- Medicine (expectorant in cough syrups)
- Glue (plywood bonding)
- Leclanche cells (electrolyte)
- Food additive (yeast nutrient in bread making)
- Acidifier
- Cooling baths (low temperature)
- Buffer solutions (with ammonia)
- Cattle feed supplement
- Health Effects
- Potential for overdosing
- Increased blood pressure
- Irritation, shortness of breath, cough, nausea, headache (ammonium chloride poisoning)
- Serious eye discomfort (gas exposure)
- Chronic exposure: asthma-like reaction, impaired kidney function
- Other
- Systemic acidifier (treatment of metabolic alkalosis)
- Oral acid loading test (identify distal renal tubular acidosis)
- Maintain acidic urine pH (urinary tract treatment)
RELATED POSTS
FAQs
Consider asking about the main topic, subtopics, connections, associations, implications, and potential actions related to the subject matter.
A central idea or topic, branches representing main themes or concepts, and interconnected sub-branches detailing related information or ideas.
It depends on the context. It might be considered good for some colleges or courses, but for others, it may fall short of expectations.
A score of 150 is generally considered quite good in JEE Mains, potentially qualifying for admission to various reputable colleges depending on the year’s cutoffs and competition.
This was all about the “Mind map for JEE”. For more such informative blogs, check out our Engineering Exams Section, or you can learn more about us by visiting our Indian exams page.


 One app for all your study abroad needs
One app for all your study abroad needs











 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!