Answer: Schiff reagent reacts with aldehydes to form a magenta or pink colour. It contains decolourised fuchsin (rosaniline hydrochloride) treated with sulfur dioxide, which is colourless. When an aldehyde is introduced, it restores the dye’s colour through the formation of a complex, indicating the presence of an aldehyde group. This reaction is used in the Schiff test for aldehydes.
Complete Answer:
Schiff reagent is a chemical test commonly used in school and college chemistry labs to detect aldehyde functional groups in a compound. The reagent itself is prepared by treating a solution of fuchsin dye (a purple-red dye) with sulfur dioxide, which removes the colour, making the solution almost colourless or pale.
Schiff reagent reacts with an aldehyde to give a pink or magenta colour. Schiff reagent is a colourless chemical solution that helps detect the presence of aldehyde groups. When an aldehyde is present, the reagent reacts and changes colour, turning pink or magenta. This visible colour change is a clear sign that an aldehyde is in the sample.
Now, here is where the chemistry gets interesting. When an aldehyde is added to this colourless Schiff reagent, something noticeable happens; it restores the colour of the dye, turning the solution pink, reddish-purple, or magenta, depending on the aldehyde used.
How Does Schiff Agent React with an Aldehyde?
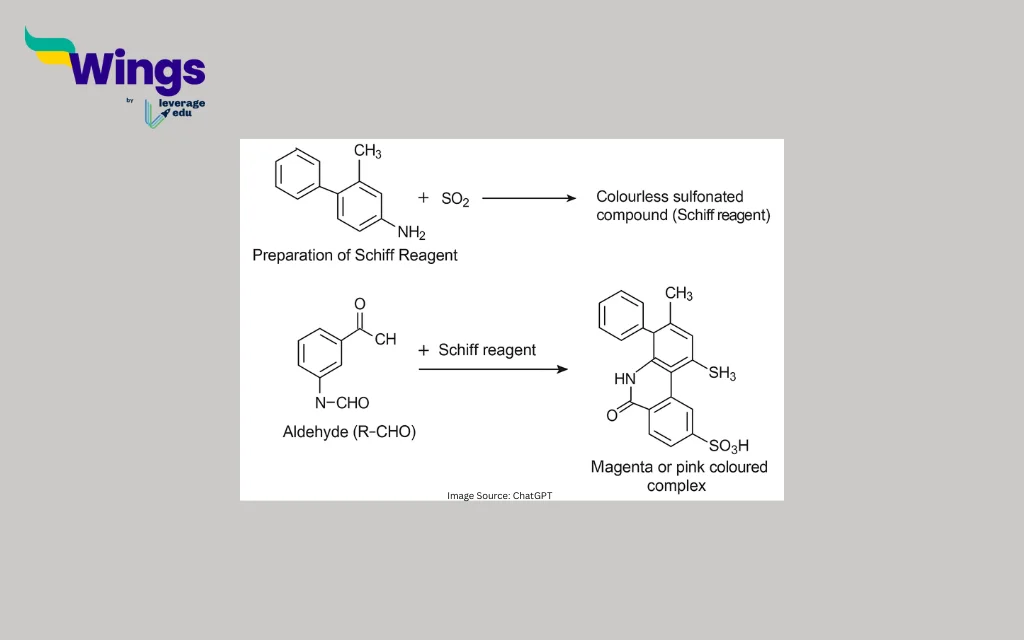
When an aldehyde (R–CHO) is added to the colourless Schiff reagent, it reacts with the decolourised dye. The aldehyde reduces the sulfonated compound formed during the preparation of the Schiff reagent, restoring its original pink or magenta colour.
Simplified Reaction Sequence
Here is the simplified reaction sequence that explains how a Schiff reagent reacts with an aldehyde:
Preparation of Schiff Reagent:
Fuchsin dye + SO₂ → Colourless sulfonated compound (Schiff reagent)
Reaction with Aldehyde:
Aldehyde (R–CHO) + Schiff reagent → Magenta or pink coloured complex
The colour change happens in the reaction because aldehydes are strong reducing agents. When they react with the Schiff reagent, they reverse the decolourisation caused by sulfur dioxide, bringing back the original colour. This specific reaction doesn’t occur with most ketones, making it a useful test to distinguish between aldehydes and ketones.
For example, if you test formaldehyde (a simple aldehyde) with Schiff reagent, the pink colour appears very quickly. On the other hand, acetone (a ketone) usually shows no colour change, making this test effective for identifying functional groups.
This test is simple yet powerful. It is widely used in basic organic chemistry experiments and also in biological staining techniques, such as the Periodic acid-Schiff (PAS) stain used to highlight structures in cells and tissues.
So, just like how bile helps in breaking down fats at the right time, Schiff reagent helps in identifying aldehydes quickly and clearly, supporting our understanding of chemical structures and their reactions.
Check some Chemistry questions from here:


 One app for all your study abroad needs
One app for all your study abroad needs










 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!