Answer: The molecular mass of benzene (C₆H₆) is 78 g/mol, making the correct option B. 78. Molecular mass is the sum of the atomic masses of all atoms in a molecule. It’s expressed in grams per mole (g/mol) and helps in determining how much of a substance is needed for chemical reactions.
Explanation
How Is the Molecular Mass of Benzene Calculated?
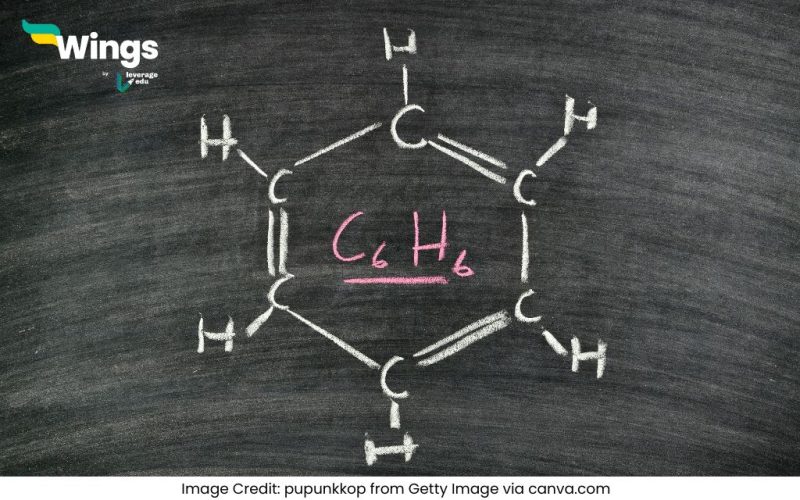
Benzene is a simple aromatic hydrocarbon with the chemical formula C₆H₆. To calculate its molecular mass:
- Carbon (C): Atomic mass = 12 g/mol. For 6 atoms: 12×6=72g/mol.
- Hydrogen (H): Atomic mass = 1 g/mol. For 6 atoms: 1×6=6g/mol.
- Total Molecular Mass: 72+6=78g/mol.
What is Benzene?
Benzene is an organic compound classified as an aromatic hydrocarbon due to its unique ring structure. It consists of six carbon atoms connected in a hexagonal ring, with one hydrogen atom attached to each carbon. Benzene is widely used in industries to produce chemicals like plastics, synthetic fibers, and resins.
So now you know that the molecular mass of Benzene is 78 g/mol. Knowing the molecular mass of substances like benzene is fundamental in chemistry. It aids in stoichiometry, reaction balancing, and preparing solutions with precise concentrations.
Common Doubts


 One app for all your study abroad needs
One app for all your study abroad needs










 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!