Answer. The IUPAC name of the following compounds, Oxalic acid is ethane-1,2-dioic acid. It is the simplest dicarboxylic acid and is commonly found in plants like spinach and rhubarb.
Complete Answer:
The correct IUPAC name of oxalic acid is ethane-1,2-dioic acid. This compound is the simplest dicarboxylic acid, with two carboxylic acid groups (-COOH) attached to a two-carbon alkane chain.
Structure and Formula of Oxalic Acid
Oxalic acid consists of two carbon atoms, and each carbon atom is bonded to a carboxylic acid group (-COOH). This gives the compound its strong acidic nature and places it in the category of dicarboxylic acids. The linear structure shows the two carboxyl groups directly connected through the carbon-carbon bond.
| Molecular Formula | C₂H₂O₄ |
| Structure of Oxalic Acid | HOOC–COOH |
Step-by-Step Explanation
Follow the steps below to determine the IUPAC name of the given compound, oxalic acid:
1. Functional Group
As told previously, oxalic acid contains two carboxylic acid (-COOH) groups, which are strong acidic functional groups. These groups play an important role in determining the chemical behaviour and reactivity of the compound.
2. Counting the Carbon Atoms
The molecule has two carbon atoms in its main chain. Therefore, the parent hydrocarbon is named ethane, which serves as the base name for compounds with two carbon atoms.
3. IUPAC Rules Implementation
According to IUPAC nomenclature, when a compound contains two carboxylic acid groups, the suffix -dioic acid is added to the name of the parent hydrocarbon. This indicates the presence of two acid groups in the molecule.
4. IUPAC Name Formation
By combining the parent name ethane with the appropriate suffix -dioic acid, the correct IUPAC name of oxalic acid becomes ethane-1,2-dioic acid.
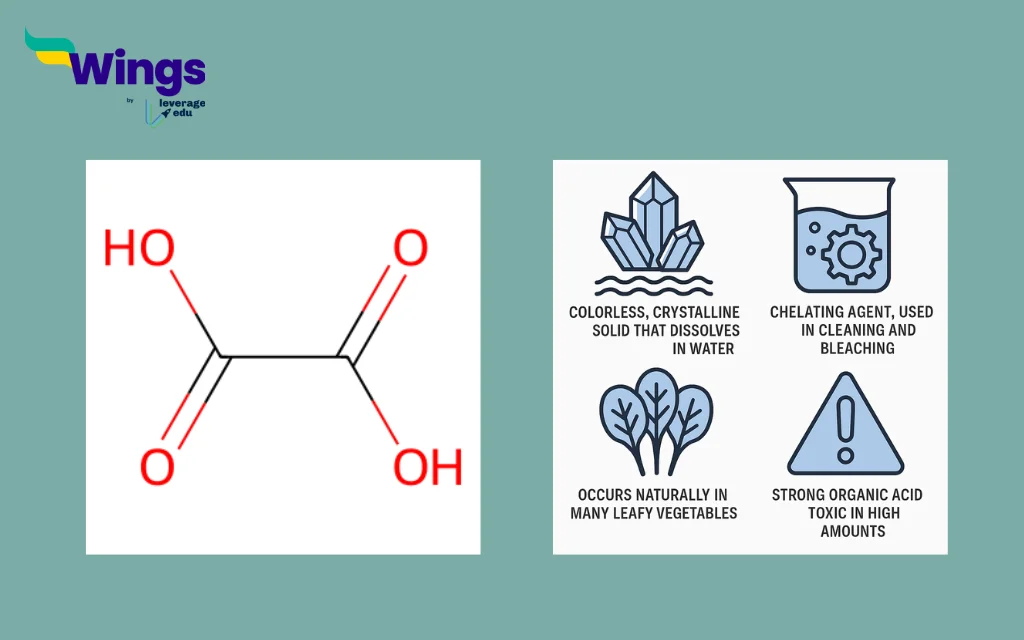
Features of Oxalic Acid
Oxalic acid is a colourless, crystalline solid that is highly soluble in water. This physical property makes it easy to handle and use in aqueous solutions.
- The function of Oxalic Acid is like a cheating agent, which means it can bind with metal ions to form stable complexes. Because of this, it is commonly used in cleaning agents and bleaching products to remove rust and stains.
- Oxalic acid occurs naturally in many leafy vegetables, such as spinach, beet greens, and rhubarb. It is a part of the plant’s defence system and contributes to its sour taste.
- The acid is highly reactive and can be toxic if consumed in large quantities. It can interfere with calcium absorption in the human body by forming insoluble calcium oxalate crystals.
Common Chemistry Doubts:


 One app for all your study abroad needs
One app for all your study abroad needs











 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!