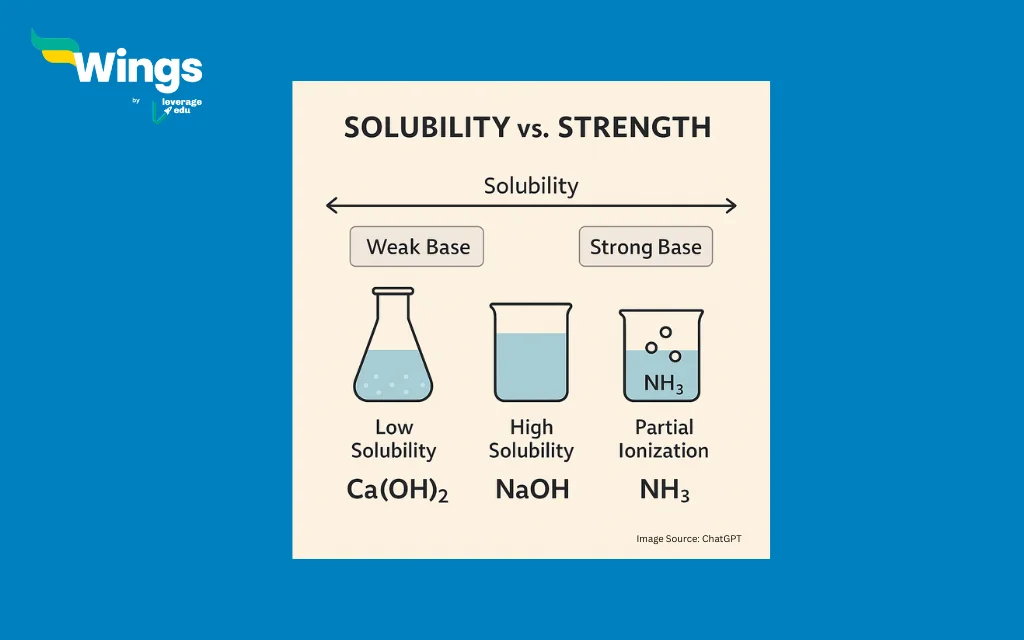
Answer: No, calcium hydroxide is not a weak alkali. Although it is only slightly soluble in water, the solution it forms fully ionises, which makes it less concentrated than strong alkalis like sodium hydroxide or potassium hydroxide, but chemically still a strong base.
Complete Answer:
Calcium hydroxide is a strong base because it ionises completely in water and breaks down into Ca²⁺ (calcium ions) and OH⁻ (hydroxide ions). However, it is not very soluble in water. This means only a small amount of it dissolves, but the part that does dissolve ionises completely. This is why it can sometimes be mistaken for a weak alkali, but chemically, it is a strong base.
- The pH of a saturated calcium hydroxide solution is around 12.4, which indicates its strong basic nature.
- Calcium hydroxide has a high pKb (base dissociation constant), which reflects that it easily gives off hydroxide ions.
- It is classified as a strong Arrhenius base, showing its contribution to OH⁻ concentration when dissolved.
Facts to Remember about Calcium Hydroxide:
- Calcium hydroxide is a strong base used in whitewashing walls and neutralising acidic soils.
- It is part of the strong bases called alkaline earth metal hydroxides.
- Complete ionisation of calcium hydroxide means that it can easily deprotonate weak acids and raise pH effectively.
Thus, calcium hydroxide is not a weak alkali; instead, it is a strong base with low solubility. Once it is dissolved, it behaves powerfully and reflects its chemical strength, even if it appears mild at first glance.
Common Chemistry Doubts:


 One app for all your study abroad needs
One app for all your study abroad needs











 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!