1. 0
2. 1
3. 2
4. 3
Correct Answer: 4) 3
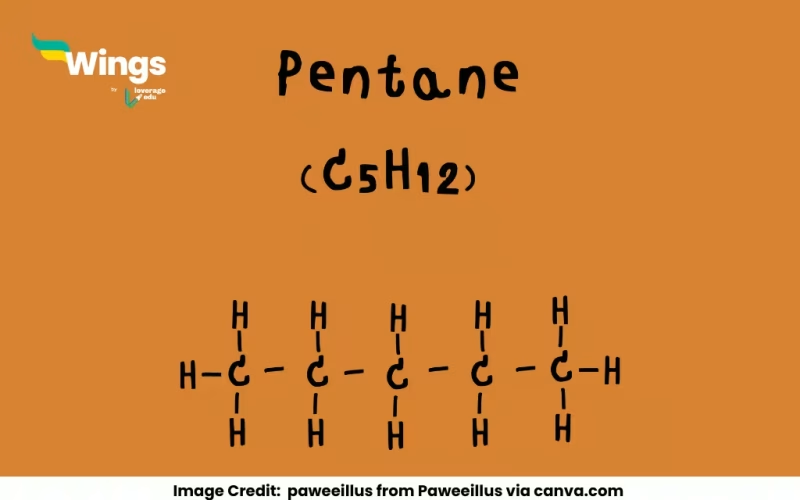
Pentane (C₅H₁₂) has three structural isomers: n-pentane, isopentane, and neopentane, each with a unique carbon arrangement. These isomers showcase how molecular structure affects properties like boiling points, making them a key concept in organic chemistry. Understanding them helps students grasp the creativity and logic of molecular design.
What Are Structural Isomers?
Let’s kick things off with the basics: what the heck are structural isomers? These are molecules with the same molecular formula but different arrangements of atoms, kind of like rearranging furniture in a room to get a new vibe. For pentane, with the formula C₅H₁₂, there are three structural isomers, meaning three unique ways to connect its five carbon atoms and twelve hydrogens.
Pentane is a hydrocarbon, part of the alkane family, where carbons are linked by single bonds. The three isomers are n-pentane (a straight chain), isopentane (a branched chain), and neopentane (a highly branched structure). For students, understanding isomers is like solving a molecular puzzle, it shows how small changes in structure can lead to different properties.
Meet the Three Isomers of Pentane
Alright, let’s introduce the trio! First up is n-pentane, where all five carbons are in a straight line, like a conga line of atoms. Its structure is CH₃-CH₂-CH₂-CH₂-CH₃. Next, we have isopentane (or 2-methylbutane), where one carbon branches off, giving it a shape like CH₃-CH(CH₃)-CH₂-CH₃. Finally, neopentane (or 2,2-dimethylpropane) is super compact, with a central carbon bonded to four methyl groups: C(CH₃)₄.
Each isomer has distinct physical properties, like boiling points, because their shapes affect how they interact. For example, n-pentane boils at 36°C, while neopentane, being more compact, boils at 9.5°C. For chemistry students, drawing these structures is a great way to visualize how carbon’s flexibility creates variety in molecules.
Why there are only Three Isomers of Pentane?
You might be wondering: why just three isomers for pentane? It’s all about the ways you can arrange five carbon atoms while keeping the alkane rules (single bonds, C₅H₁₂ formula). Carbon can form four bonds, so you start with a straight chain, then try branching one carbon, then two, and so on. After testing all combos, only three unique structures work without repeating or breaking the formula.
This limit is a cool lesson in combinatorial chemistry. For smaller alkanes, like butane, you get fewer isomers (two), but as the carbon count grows, the possibilities explode. For example, hexane (C₆H₁₄) has five isomers. Students can use this to practice systematic thinking, like mapping out all possible structures to confirm the count.
Quick Facts
- Number of Isomers: 3 (n-pentane, isopentane, neopentane).
- Formula: C₅H₁₂.
- Type: Alkane (single-bonded hydrocarbon).
- Key Use: Fuels, solvents.
- Boiling Points: Vary due to structural differences.
Importance of Pentane and Its Isomers in Chemistry
Isomers aren’t just academic trivia—they matter in the real world. Different isomers of pentane have different uses, like in fuels or solvents, because their properties vary. For instance, isopentane is used in some industrial processes due to its lower boiling point. Understanding isomers helps chemists design molecules for specific jobs.
For students, isomers also show why structure matters in chemistry. A tiny tweak in how atoms are arranged can change a molecule’s behavior, which is critical in fields like drug design or materials science. It’s a reminder that chemistry is as much about creativity as it is about rules.
How to Identify Isomers?
Want to find pentane’s isomers yourself? Start by drawing n-pentane’s straight chain. Then, try moving one carbon to form a branch, which gives isopentane. Next, branch two carbons off a central one for neopentane. You can use a pencil and paper or molecular model kits to play around. The key is ensuring each structure has exactly 5 carbons and 12 hydrogens.
A handy tip for students: check for duplicates. Sometimes, what looks like a new isomer is just a rotated version of another. Also, name each isomer using IUPAC rules (like 2-methylbutane for isopentane) to confirm they’re distinct. This exercise sharpens your organic chemistry skills and makes you appreciate carbon’s versatility.
Fun Facts About Pentane
Pentane itself is pretty cool beyond its isomers. It’s a liquid at room temperature, found in crude oil, and used as a solvent or fuel component. Its isomers pop up in gasoline blends, affecting how efficiently fuel burns. Neopentane, with its compact shape, is even used in some lab experiments as a reference molecule.
For students, pentane’s isomers are a gateway to organic chemistry’s bigger picture. Once you nail pentane’s three isomers, you’re ready to tackle more complex molecules. Plus, it’s satisfying to know you’ve cracked a molecular code that’s part of everyday products like fuel!
Summary
Question: How many Structural Isomers are Possible for Pentane?
Answer: There are 3 Structural Isomers are possible for Pentane, which are n-pentane, isopentane, and neopentane. The three isomers of pentane are like a mini-lesson in chemistry’s magic: same formula, different personalities. From n-pentane’s straight chain to neopentane’s compact blob, these structures show how small tweaks create big differences. For students, mastering pentane’s isomers is a fun step toward unlocking the wild world of organic chemistry, so grab a pencil and start drawing!
Also Read:
What is Blue Vitriol?
Which Indian Cricketer is also known as Little Master?
Where is ‘Dudhsagar Waterfall’ situated in India
Panchavti, a Key Part of the Valmiki Ramayana is located in which State of
India?
The Longest River in India is


 One app for all your study abroad needs
One app for all your study abroad needs










 60,000+ students trusted us with their dreams. Take the first step today!
60,000+ students trusted us with their dreams. Take the first step today!