Lanthanoids and Actinoids are two series of elements in the periodic table. Lanthanoids (elements 57-71) belong to the f-block and are known for their shiny, soft metals, often used in magnets and lasers. Actinoids (elements 89-103) are radioactive, with some used in nuclear energy. Both series fill the 4f and 5f orbitals, respectively. Keep reading to learn how to differentiate between Lanthanoids and Actinoids.
Complete Answer:
Lanthanoids and Actinoids are f-block elements, but they differ in key aspects. Lanthanoids (elements 57-71) involve the filling of 4f orbitals, are mostly non-radioactive, and show limited oxidation states (+3 being common). They are used in magnets, phosphors, and catalysts. Actinoids (elements 89-103) involve 5f orbital filling, are all radioactive, and exhibit variable oxidation states (+3, +4, +5, etc.). While lanthanoids occur naturally, most actinoids are synthetic and are used in nuclear energy and medicine.
Here is how to differentiate between Lanthanoids and Actinoids:
| Lanthanoids | Actinoids |
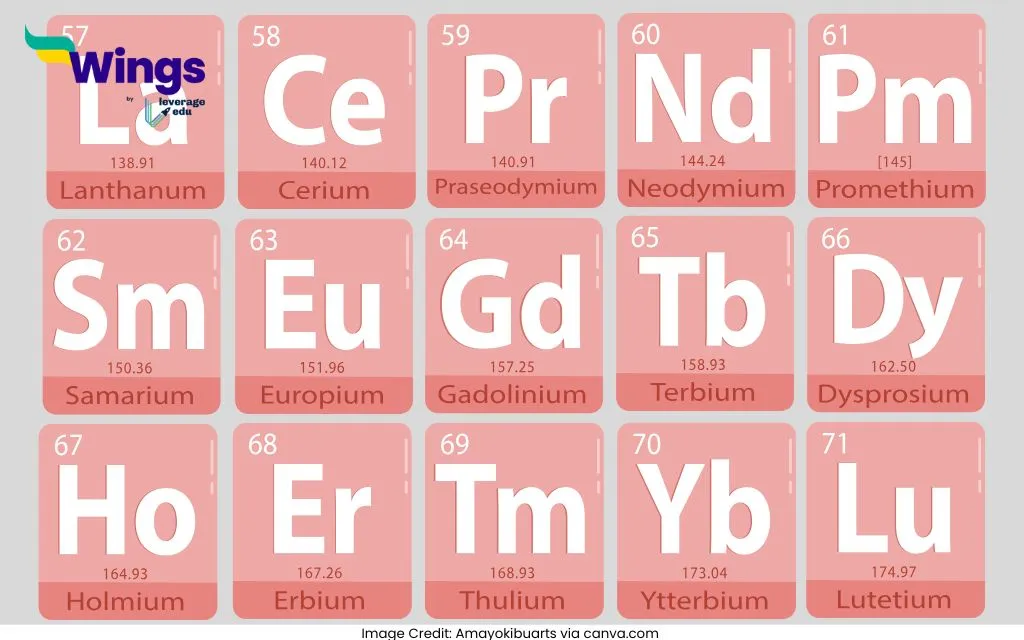
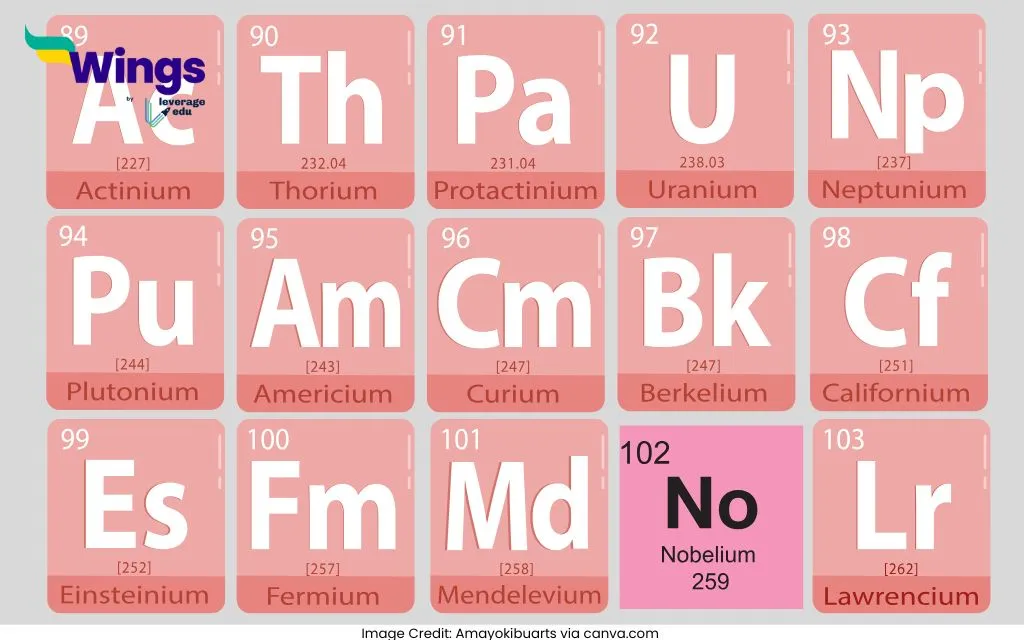
| The atomic numbers of Lanthanoid elements range from 57 to 71. Here is list of Lanthanoids: Lanthanum (La) – 57 Cerium (Ce) – 58 Praseodymium (Pr) – 59 Neodymium (Nd) – 60 Promethium (Pm) – 61 Samarium (Sm) – 62 Europium (Eu) – 63 Gadolinium (Gd) – 64 Terbium (Tb) – 65 Dysprosium (Dy) – 66 Holmium (Ho) – 67 Erbium (Er) – 68 Thulium (Tm) – 69 Ytterbium (Yb) – 70 Lutetium (Lu) – 71 | The atomic numbers of Lanthanoid elements range from 89 to 103. Here is a list of Actinoids: Actinium (Ac) – 89 Thorium (Th) – 90 Protactinium (Pa) – 91 Uranium (U) – 92 Neptunium (Np) – 93 Plutonium (Pu) – 94 Americium (Am) – 95 Curium (Cm) – 96 Berkelium (Bk) – 97 Californium (Cf) – 98 Einsteinium (Es) – 99 Fermium (Fm) – 100 Mendelevium (Md) – 101 Nobelium (No) – 102 Lawrencium (Lr) – 103 |
| These elements fill the 4f orbitals progressively. | These elements fill the 5f orbitals progressively. |
| Most lanthanoids are non-radioactive, except for a few isotopes. | All actinoids are radioactive. |
| Lanthanoids are found in the Earth’s crust. | Most of the Actinoids; except elements like uranium, thorium, etc; are synthetic. |
| These elements have a common oxidation state of +3, with limited variability. | These elements show variable oxidation states (+3, +4, +5, etc.). |
| Lantanoids are less reactive than actinoids. Their reactivity increases with atomic number. | Actinoids are highly reactive, especially with oxygen and halogens. |
| Lanthanoid elements are usually harder and have higher melting points than actinoids. | Actinoids are softer and have relatively lower melting points than Lanthanoids. |
| These elements show strong paramagnetism. | These elements are paramagnetic but influenced by radioactive properties. |
| Lanthanoids are used in magnets, phosphors, and catalysts. | Actinoids are used in nuclear reactors, weapons, and medicine. |
Interesting Facts about Lanthanoids and Actinoids
Here are some interesting facts about Lanthanoids:
- Lanthanoids are often called “rare earth metals” and have a silvery appearance, but they tarnish quickly in air.
- Lanthanoids like Neodymium (Nd) are used to make powerful magnets found in electric motors and headphones.
- Europium (Eu) and Terbium (Tb) are used in phosphors for TV screens and fluorescent lights.
- Promethium (Pm) is the only lanthanoid that does not occur naturally and is produced in nuclear reactors.
- Lanthanoids are highly reactive with water and acids, releasing hydrogen gas.
Here are some interesting facts about Actinoids:
- All actinoids are radioactive, and many are highly unstable, decaying into other elements.
- Uranium (U) and Plutonium (Pu) are critical in nuclear power and weapons development.
- Most actinoids, like Americium (Am) and Curium (Cm), are synthetic and do not exist naturally.
- Actinium (Ac) is used in cancer treatments due to its radioactive properties.
- Actinoids react readily with oxygen, halogens, and acids, forming a variety of compounds.
Common Chemistry Questions:
 45,000+ students trusted us with their dreams. Take the first step today!
45,000+ students trusted us with their dreams. Take the first step today!


 One app for all your study abroad needs
One app for all your study abroad needs